Thin-wall technology expands radial access for more patients
•Perform diagnostic and interventional procedures without upsizing to a larger sheath
•Provide easy radial access, especially in women with smaller radial arteries1,3
•A smaller diameter sheath reduces the arteriotomy size, to enhance post-procedure haemostasis1

Thin-wall technology expands radial access for more complex PCI
5Fr for invasive PCI for patients with small radial artery diameter
6Fr as standard size for daily practice PCI
7Fr for complex PCI & larger devices (rotablation, bifurcation, left main, CTO, etc.)
MCoat™: Terumo’s hydrophilic coating
Ease of insertion and removal
Reduce penetration resistance compared to conventional sheaths4
Reduce friction between the vessel and the introducer2
Lower risk of adverse events such as radial artery occlusion1,2
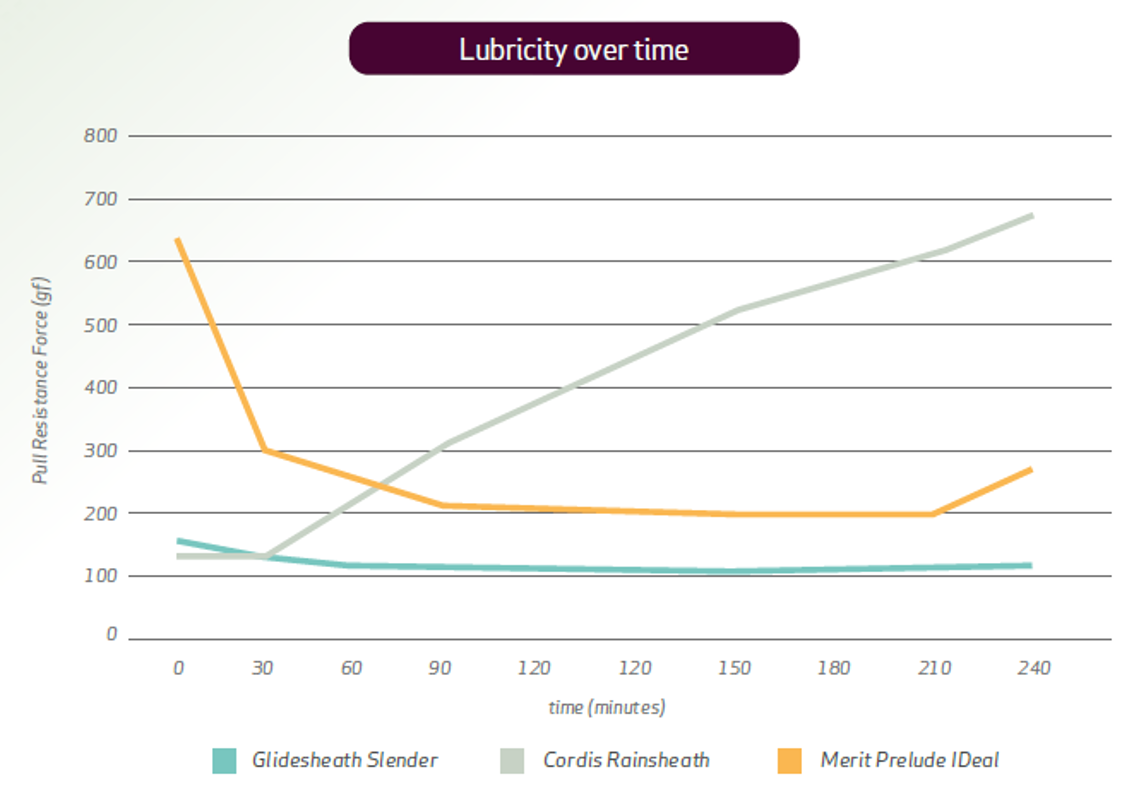
Glidesheath Slender™ demonstrates superior and consistent lubricity, and lubricity durability over time5
Item specifications
This kit contains sheath, dilator, plastic or spring mini guidewire, plastic IV catheter (entry needle) or a metallic entry needle.
Outer Diameter
|
Sheath Length
|
Entry Needle-Diameter
|
Entry Needle-Length
|
Entry Needle-Type
|
Mini Guidewire-Type
|
Mini Guidewire-Diameter
|
Mini Guidewire-Length
|
Units/Box |
Code |
| 5 Fr | 10 cm
| 20 G
0.9 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.025 in
0.64 mm
| 45 cm
| 5
| RM*RS5J10PQ
|
5 Fr
| 10 cm
| 20 G
0.9 mm
| 51 mm
| Plastic IV Catheter
| Plastic
| 0.025 in
0.64 mm
| 45 cm
| 5
| RM*ES5J10SQ
|
5 Fr
| 10 cm
| 21 G
0.8 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.021 in
0.53 mm
| 45 cm | 5
| RM*RS5F10PQ
|
5 Fr
| 10 cm
| 22 G
0.7 mm
| 32 mm
| Plastic IV Catheter
| Plastic
| 0.021 in
0.53 mm
| 45 cm
| 5
| RM*ES5F10SQR
|
5 Fr
| 10 cm
| 22 G
0.7 mm
| 35 mm
| Entry Needle
| Spring
| 0.018 in
0.46 mm
| 45 cm
| 5
| RM*RS5C10PQ
|
5 Fr
| 16 cm
| 21 G
0.8 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.021 in
0.53 mm
| 80 cm
| 5
| RM*RS5F16PQ
|
6 Fr
| 10 cm | 20 G
0.9 mm
| 32 mm
| Plastic IV Catheter
| Plastic Shortangle
| 0.025 in 0.64 mm
| 45 cm
| 5
| RM*ES6J10HQS
|
6 Fr
| 10 cm
| 20 G
0.9 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.025 in 0.64 mm
| 45 cm | 5
| RM*RS6J10PQ
|
6 Fr
| 10 cm
| 20 G
0.9 mm
| 51 mm
| Plastic IV Catheter
| Plastic
| 0.025 in 0.64 mm
| 45 cm
| 5
| RM*ES6J10SQ
|
6 Fr
| 10 cm
| 20 G
0.9 mm
| 51 mm
| Plastic IV Catheter
| Spring
| 0.025 in 0.64 mm
| 45 cm
| 5
| RM*ES6J10PQ
|
6 Fr
| 10 cm
| 21 G 0.8 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.021 in 0.53 mm
| 45 cm
| 5
| RM*RS6F10PQ
|
6 Fr
| 10 cm
| 22 G
0.7 mm
| 25 mm
| Plastic IV Catheter
| Plastic
| 0.021 in 0.53 mm
| 45 cm | 5 | RM*ES6F10SQ
|
6 Fr
| 10 cm
| 22 G
0.7 mm
| 32 mm
| Plastic IV Catheter
| Plastic
| 0.021 in 0.53 mm
| 45 cm
| 5
| RM*ES6F10SQR
|
6 Fr
| 10 cm
| 22 G
0.7 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.018 in 0.46 mm
| 45 cm
| 5
| RM*RS6C10PQ |
6 Fr
| 16 cm
| 20 G 0.9 mm
| 32 mm | Plastic IV Catheter
| Plastic Shortangle
| 0.025 in 0.64 mm
| 80 cm
| 5
| RM*ES6J16HQS
|
6 Fr
| 16 cm
| 20 G
0.9 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.025 in 0.64 mm | 80 cm
| 5
| RM*RS6J16PQ
|
6 Fr
| 16 cm
| 20 G 0.9 mm
| 51 mm
| Plastic IV Catheter
| Plastic
| 0.025 in 0.64 mm
| 80 cm
| 5
| RM*ES6J16SQ
|
6 Fr
| 16 cm
| 21 G
0.8 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.021 in 0.53 mm
| 80 cm
| 5
| RM*RS6F16PQ
|
7 Fr
| 10 cm
| 20 G
0.9 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.025 in 0.64 mm
| 45 cm
| 5
| RM*RS7J10PQ
|
7 Fr
| 10 cm
| 20 G 0.9 mm
| 51 mm
| Plastic IV Catheter
| Plastic
| 0.025 in 0.64 mm
| 45 cm
| 5
| RM*ES7J10SQ
|
7 Fr
| 10 cm
| 21 G 0.8 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.021 in 0.53 mm
| 45 cm
| 5
| RM*RS7F10PQ
|
7 Fr
| 10 cm
| 22 G
0.7 mm
| 32 mm
| Plastic IV Catheter
| Plastic
| 0.021 in 0.53 mm
| 45 cm
| 5
| RM*ES7F10SQR
|
7 Fr
| 16 cm
| 20 G
0.9 mm
| 32 mm
| Plastic IV Catheter
| Plastic Shortangle
| 0.025 in 0.64 mm
| 80 cm
| 5
| RM*ES7J16HQS
|
7 Fr
| 16 cm
| 20 G
0.9 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.025 in 0.64 mm
| 80 cm
| 5
| RM*RS7J16PQ
|
7 Fr
| 16 cm
| 21 G
0.8 mm
| 35 mm
| Metallic Entry Needle
| Spring
| 0.021 in 0.53 mm
| 80 cm
| 5
| RM*RS7F16PQ
|
7 Fr
| 16 cm
| 22 G
0.7 mm
| 32 mm
| Plastic IV Catheter
| Plastic
| 0.021 in 0.53 mm
| 80 cm
| 5
| RM*ES7F16SQR
|
Please quote above item reference codes when placing an order
References
1. Rao S. et al. Euro Heart J. 2012; 33(20): 2521-2526.
2. Saito S. et al. Cath Cardio Inter. 2002; 56(3): 328-332.
3. Saito S. et al. Catheter Cadiovasc Interv. 1999; 46(2):173-178.
4. Kiemeneij F. et al. Catheter Cardiovasc Interv 2003;59:161–164
5. Terumo’s Data on file – ISCD – 123-32-60
