Background
Supported by compelling evidence, conventional TransRadial Access (TRA) and the radial first strategy are now advocated as the default approach by both the ESC/EACTS & ACC/AHA/SCAI Guidelines. Prevention of Radial Artery Occlusion (RAO), the most frequent complication of TRA, is becoming a central consideration for achieving a successful radial program. However, real-world incidence of RAO remains high. Distal Radial Access (DRA) has emerged as a promising alternative to conventional TRA, to help further reduce RAO.
Objective
The aim of the DISCO RADIAL trial is to assess the superiority of DRA compared to conventional TRA with respect to the incidence of forearm RAO at discharge, while systematically implementing best practices to reduce RAO.
Methods
The DISCO RADIAL trial is a prospective, multicenter, international, open label, randomized, controlled study involving experienced operators qualified for TRA & DRA procedures.
Radial artery occlusion preventive measures included:
- Adequate anticoagulation
- Effective spasmolytic treatment
- Glidesheath Slender™ 6Fr sheath, as the default sheath for both TRA and DRA to exclude the variation in access devices as confounding factor.
- Patent hemostasis protocol from the PROPHET study, using TR Band™ for TRA patient, while hemostasis was per hospital practice for DRA patients
- Extensive radial expertise
The primary endpoint was the incidence of forearm RAO at hospitalization discharge, assessed by an independent investigator not involved in the procedure. For DRA patients, occlusion of both forearm and distal arteries were assessed.
The secondary endpoints included:
- Vascular access phase: sheath insertion time, cross-over*, radial artery spasm
- Coronary procedure phase: procedure duration, radiation dose
- Hemostasis phase: use of inflatable device, patent hemostasis (only in TRA group), time to hemostasis
- Complications: distal artery occlusion (only in DRA group), overall bleedings** & puncture site bleedings***, vascular access-site complications, radial artery spasm, access-related pain****
* If the initial attempt to obtain vascular access at the randomized access site (DRA or TRA) failed, all further attempts were considered as cross-over. This included the use of the contralateral arm, other arteries or cross-over to the other group
**Defined according to the Bleeding Academic Research Consortium (BARC) criteria
*** Defined according to the Early Discharge After Transradial Stenting of Coronary Arteries Study (EASY) criteria
**** Rated according to self-reported visual analogue scale (VAS)
Patient Population
A total of 1307 patients were randomized in the ITT (Intention-To-Treat) population: 657 in the TRA group & 650 in the DRA group. Patient enrolment aimed for a population representative of routine clinical practice. The percentages below represent the baseline patient characteristics among both groups:
- 68.1 years old male patient (72.5%)
- Comorbidities:
- Arterial hypertension (78.2%)
- Hyperlipidemia (69.9%)
- Cardiovascular history (23% history of MI, 38.3% with previous PCI, 84.9% with chronic coronary syndrome)
- Antiplatelet medication (99.4%)
- Procedural needs:
- 63% diagnostic angiography only
- 37% PCI procedure, requiring on average 1.5 stent
Primary Endpoint Result: Rao at Discharge
- Exceptionally low incidence of RAO at discharge in both groups, the lowest ever reported in any multicenter TRA trial. Those results support clinical application of best practice recommendations for the reduction of RAO.
- Patent hemostasis was achieved in 94.4% of patient in the TRA group.
- Distal RAO in the DRA group was 0.46%.
Secondary Endpoint Results
More frequent cross over with DRA
- Cross over mainly occurred to ipsilateral conventional TRA allowing to keep on with the initial radial access strategy and its benefits over femoral access.
More frequent radial artery spasms with DRA
- Despite a higher spasm rate, patient self-reported pain rating was similar in both groups.
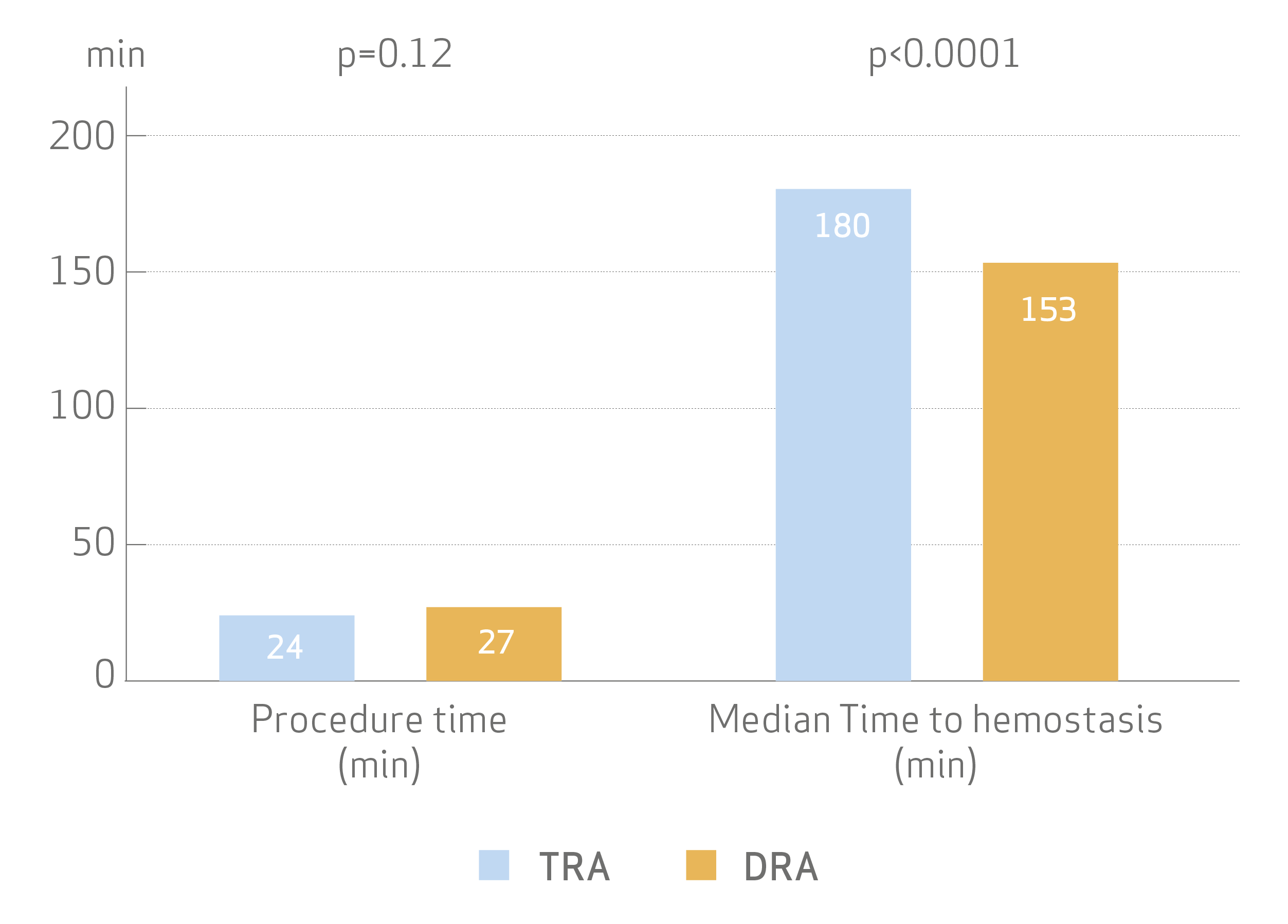
Procedural time was not significantly different, but median time to hemostasis was shorter with DRA
- Time to successfully achieve sheath insertion for DRA was only slightly longer (1 vs 2 min, p<0.001).
- No differences were seen in procedural parameter including the use of contrast medium and radiation dose.
No difference in bleedings, vascular complications, or access-related pain between TRA & DRA
- Patient self-reported pain rating was similar in both groups (2 vs 2, p=0.067)
Conclusion
DISCO RADIAL is the first large international randomized clinical trial to -
Provide information on current practice of DRA in experienced radial centers
Support distal radial access as a valid alternative to conventional radial access
Demonstrate equally low forearm RAO rates with conventional & distal radial access, highlighting the importance and clinical benefits of a rigorous hemostasis protocol.
DISCO RADIAL is also the first large international trial implementing best practice recommendations for the reduction of RAO after conventional TRA, including the use of
- Glidesheath Slender™ to apply the lowest profile system necessary to complete the procedure.
- TR Band™ to achieve non occlusive patent hemostasis, with minimal pressure strategy together with short hemostasis time.
DISCO RADIAL establishes a new reference in transradial practice for radial artery occlusion prevention, with the lowest ever reported forearm RAO in any multicenter TRA trial, confirming TRA as the gold standard for vascular access.
Link to full publication: https://www.sciencedirect.com/science/article/pii/S1936879822008974
