 Terumo Europe N.V. is pleased to announce that its K-Pack Surshield™ needle was selected for purchase by a global pharmaceutical company and first commercial shipments of the device have already begun.
Terumo Europe N.V. is pleased to announce that its K-Pack Surshield™ needle was selected for purchase by a global pharmaceutical company and first commercial shipments of the device have already begun.
The customer, whose identity remains confidential at this stage, plans to market the innovative safety needle together with drugs for applications in fields such as hepatitis and liver transplantation. In particular, patients who self inject medications are expected to benefit from this new drug and device solution.
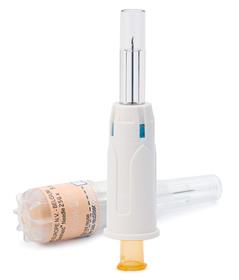
K-Pack Surshield™ is an innovative hypodermic needle with an integrated passive sharps protection feature for use with (pre)-filled syringes. The product was created with the specific needs of the pharmaceutical industry in mind. The device geometry is designed to allow for automated manipulations during packaging processes, which makes it ideal for bundling and inclusion into the secondary packaging of parenteral medication.
In the targeted applications, the customer will utilize K-Pack Surshield™ needles together with a pre-filled syringe for subcutaneous administration. This option provides patients and attending physicians with a convenient and time-saving treatment option as it allows patients to safely self- administer the drug.
The European Commission estimates that 1,200,000 needle stick injuries occur in Europe each year1. It is recognized that healthcare workers, caregivers and patients frequently risk infection due to injuries caused by needles. The consequences may be very severe, possibly leading to serious diseases such as viral hepatitis or AIDS.
In order to reduce the risk to people administering injections, Terumo has made significant investments in the development of medical devices incorporating safety-engineered protection features. Terumo is privileged to work together with this global provider of pharmaceutical and biotherapeutic drugs and particularly appreciates their responsible approach to safety. By providing the K-Pack Surshield™ safety needle with the medication, caretakers as well as the family of the patient are optimally protected against the risks of bloodborne pathogens associated with needle stick injuries.
Developed for subcutaneous and intra-muscular injections, Terumo's K-Pack Surshield™ hypodermic needle is equipped with passive sharps protection. The encapsulating protection feature is integrated into the needle, whereby the needle is never exposed and the protection mechanism is 'armed' during insertion to prevent the system from being bypassed. The needle fits to any Luer syringe and as the protection feature is integrated into the needle, the system does not affect the performance of the syringe.
The customer has indicated that it is considering K-Pack Surshield™ for other projects in its pipeline and has shown interest in continued collaboration with Terumo on innovative projects in the future.
About Terumo
Tokyo-based Terumo Corporation is one of the world's leading medical device manufacturers with $4 billion in sales and operations in more than 160 nations. Since its founding in 1921, Terumo Corporation has based its business activities on a corporate philosophy of "Contributing to Society through Healthcare". The company pursues global business development designed to improve the quality of healthcare for people all around the world.
With decades of expertise and core competences in parenteral drug delivery and injection technology, Terumo continuously strives to research and develop advanced, innovative devices. This is underpinned by the relentless pursuit of biocompatibility and technologies centered on materials technology driven by each product's distinct characteristics and technological aptitude in production capabilities.
Terumo Corporation's shares are listed on the first section of the Tokyo Stock Exchange and is a component of the Nikkei, Japan's leading stock index.
(1) Reference: Commission of the European Communities, Proposal for a Council Directive: Implementing the Framework Agreement on prevention from sharp injuries in the hospital and healthcare sector concluded by HOSPEEM and EPSU (October 2009). http://eur-lex.europa.eu
For more information, contact Kristina Maes, Terumo Europe Global Pharmaceutical Solutions, +32 1638 1500, kristina.maes@terumo-europe.com.
###
