Background
Transarterial chemoembolization (TACE) using irinotecan-eluting beads is an additional treatment option for patients with colorectal cancer liver metastases (CRLM) not eligible for curative treatment approaches.
Objective:
This interim analysis focuses on feasibility of the planned statistical analysis regarding data distribution and completeness, treatment intention, safety and health-related quality of life (HRQOL) of the first 50 patients prospectively enrolled in the CIRSE Registry for LifePearlTM microspheres (CIREL), an observational multicentre study conducted across Europe.
Methods
Prospective, observational multicentre real-life study conducted across Europe
In total, 50 patients >18 years diagnosed with CRLM and deemed to be treated with irinotecan-eluting LifePearlTM microspheres TACE (LP-irinotecan TACE) by a multidisciplinary tumour board received 129 TACE sessions. There were no further inclusion or exclusion criteria.
The primary endpoint was the categorisation of treatment intention; secondary endpoints presented in this interim analysis are safety, treatment considerations and HRQOL
Results
Pereira et al registry (n=50) demonstrated:
LP TACE irinotecan Treatment intentions
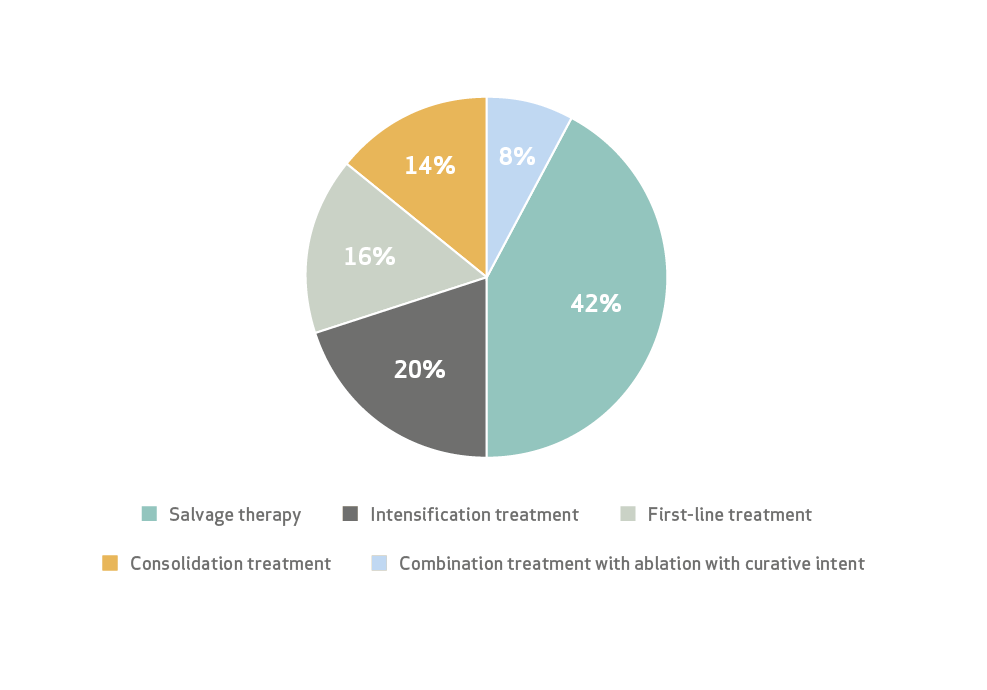
Salvage therapy was considered for progressive disease after 3 or more lines of systemic therapy
Intensification therapy was proposed for patients with progressive disease that received 1 or 2 previous lines of systemic therapy.
Consolidation treatment was decided for patients that obtained stable disease during or after systemic therapy.
Technical success was 100%. 86% of TACE were performed with LP 100 micron.
Grade 3 and 4 adverse events were reported by 4% of patients during procedure and by 10% within 30 days.
While 38% reported a worse, 62% reported a stable or better global health score, and 54% of patients with worse global health score were treated as salvage therapy.
Analysing peri-procedural medications, the authors observed important differences between centres.
Limitations: although data for this interim analysis are from 50 patients from 9 centres in 7 European countries. The final data set will include patients from 25 different centres in 12 different countries. Therefore, conclusions could be subject to change as standard practices in different centres and countries could differ. Additionally, as analysing all effectiveness outcomes was beyond the scope of this interim analysis, follow-up data collection is still ongoing and the results cannot yet be analysed in conjunction with clinical outcomes such as overall survival and hepatic progression-free survival.
Conclusion
Pereira et al concluded that this interim analysis confirms in prospective the feasibility of the study, with an acceptable toxicity profile. More patients reported stable or improved HRQOL than deterioration. Deterioration of HRQOL was observed more frequently in salvage therapy patients.
Key Takeaway:
This interim analysis illustrated that in CIREL, LP irinotecan TACE was mainly used as salvage or intensification therapy with an acceptable toxicity profile.
HRQOL for the global health score deteriorated in more patients than for the functional and symptom score, for both of which over 70% of patients reported stable or improved values at the first follow-up compared to baseline.
There are different local treatment modalities available (i.e. thermal ablation, radioembolisation, intra-arterial infusion) that compete with TACE in this indication, as well as different lines of systemic therapy (therapy continuum-of-care) therefore it's important that the interventional radiologist attend the multi-disciplinary tumour board to be able to proactively propose this type of intervention.
A further standardization of peri-procedural management will eventually allow for better pain control and improvements on the impact of LP-irinotecan TACE on patients' quality of life.
Access to the full publication: https://link.springer.com/article/10.1007/s00270-020-02646-8
European Union Indications for use
LifePearl™ microspheres are indicated for embolization of blood vessels supplying primary hypervascular tumours or metastases in the liver. Note: LifePearl™ microspheres can be loaded with chemotherapeutic drugs. When used for drug loading, drug loading should be done under a physician's direction, choice and responsibility, based on type and dose of drug most beneficial to the patient. LifePearl™ microspheres are compatible with doxorubicin, epirubicin idarubicin and irinotecan. LifePearl™ microspheres can be drug loaded prior to embolization and then, as a secondary action, elute a local, controlled, and sustained dose to the targeted tumour sites after embolization. LifePearl™ microspheres are not available for sale in all countries. This information is provided only in respect to markets where this product is approved or cleared.
This literature summary is not a systemic review. It is only an example of LifePearl microspheres related literatures.
The use of the LifePearl™ devices in combination with drugs is not cleared or approved in the USA by the Food and Drug Administration. LifePearl™ microspheres are not approved in Canada. Please consult the indication of use with the IFU supplemented with the product.
Please contact your Terumo local sales representative for more information. All brand names are trademarks or registered trademarks of TERUMO CORPORATION and their respective owners. Refer to Instructions for Use for Contraindications, Warnings and Precautions.
©2021 MicroVention Europe CE0297
Manufacturer
MicroVention Europe
30 bis, rue du Vieil Abreuvoir
78100 Saint-Germain-en-Laye - France
Tel: +33(0)1 39 21 77 46
Distributor
Terumo Europe N.V.
Interleuvenlaan 40
3001 Leuven Belgium
Tel: +32 16 38 12 11
