Background
Transarterial chemoembolization (TACE) is indicated for the treatment of hepatocellular carcinoma (HCC) not amenable to curative treatments in patients with preserved liver function. Level 1 evidence indicates that TACE prolongs survival in patients with well-compensated liver disease and BCLC-B stage HCC and BCLC 0-A not suitable for options with higher priority.
Objective:
Drug eluting microspheres (DEM) loaded with anthracyclines is a variation of TACE that enables a sustained drug release. LifePearlTM microspheres is a novel DEM comprised of polyethylene-glycol with good safety profile and efficacy as reported in different studies. The purpose of this study was to assess the safety and efficacy of anthracycline-loaded LifePearl Microspheres for the treatment of patients with unresectable HCC in a pooled analysis of studies with available patient's level data from more than 500 patients.
Methods
The authors pooled patient-level data from 5 single-arm studies (3 retrospective and 2 prospective, including one pharmacokinetics study).
Safety was assessed by close monitoring of adverse events according to CTCAE (v4.03). Tumour response was evaluated following hospital practice, according to mRECIST and RECIST1.1 and analyzed as best overall response (BOR) to account for differences in imaging follow up between studies.
The Kaplan-Meier method was used to estimate event rates for time to event outcomes: progression-free survival (PFS), time to unTACEable progression (TTUP) and overall survival (OS).
Results
De Baere et al pooled analysis (n=586) demonstrated :
Out of 586 patients, 72.1% were males, with mean age of 66.8±10.3years.
At baseline, patients were 85.5% Child-Pugh A, 13.5% B and 1.0% C. The mean number of HCC lesions was 2.1±1.5 and mean sum of tumour diameters was 49.3±32.9mm. BCLC stages 0, A, B and C were 13.6%, 43.4%, 41.1% and 1.9%, respectively. The mean alpha-fetoprotein level was 680.2±4,241.0 ng/ml with 18.8% patients having > 200ng/ml.
A mean of 1.9±1.0 procedures was performed per patient.
- Adverse events (AE) were reported for 197 patients (33.6%) including 2.6% grade 4 and 1.5% grade 5. The most frequent AE were related to post-embolization syndrome.
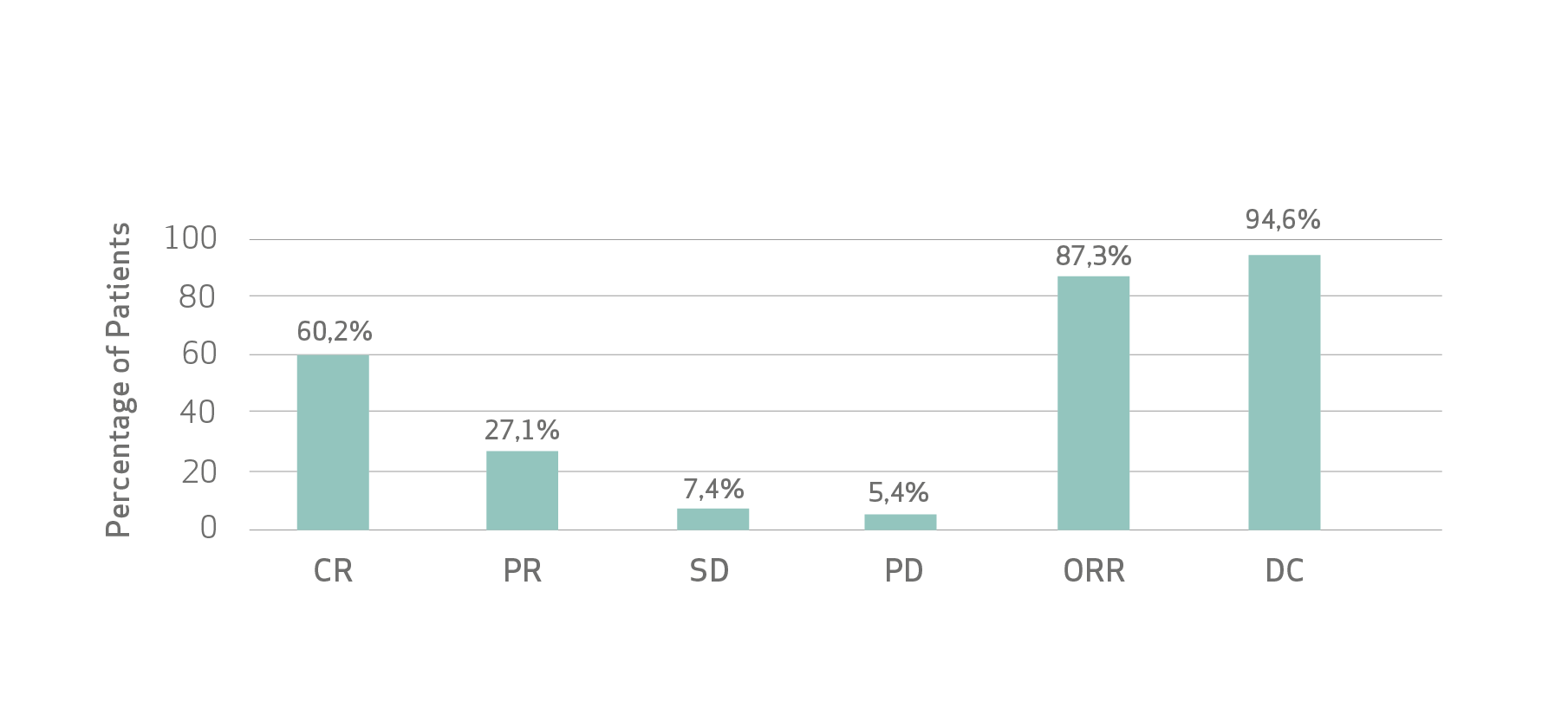
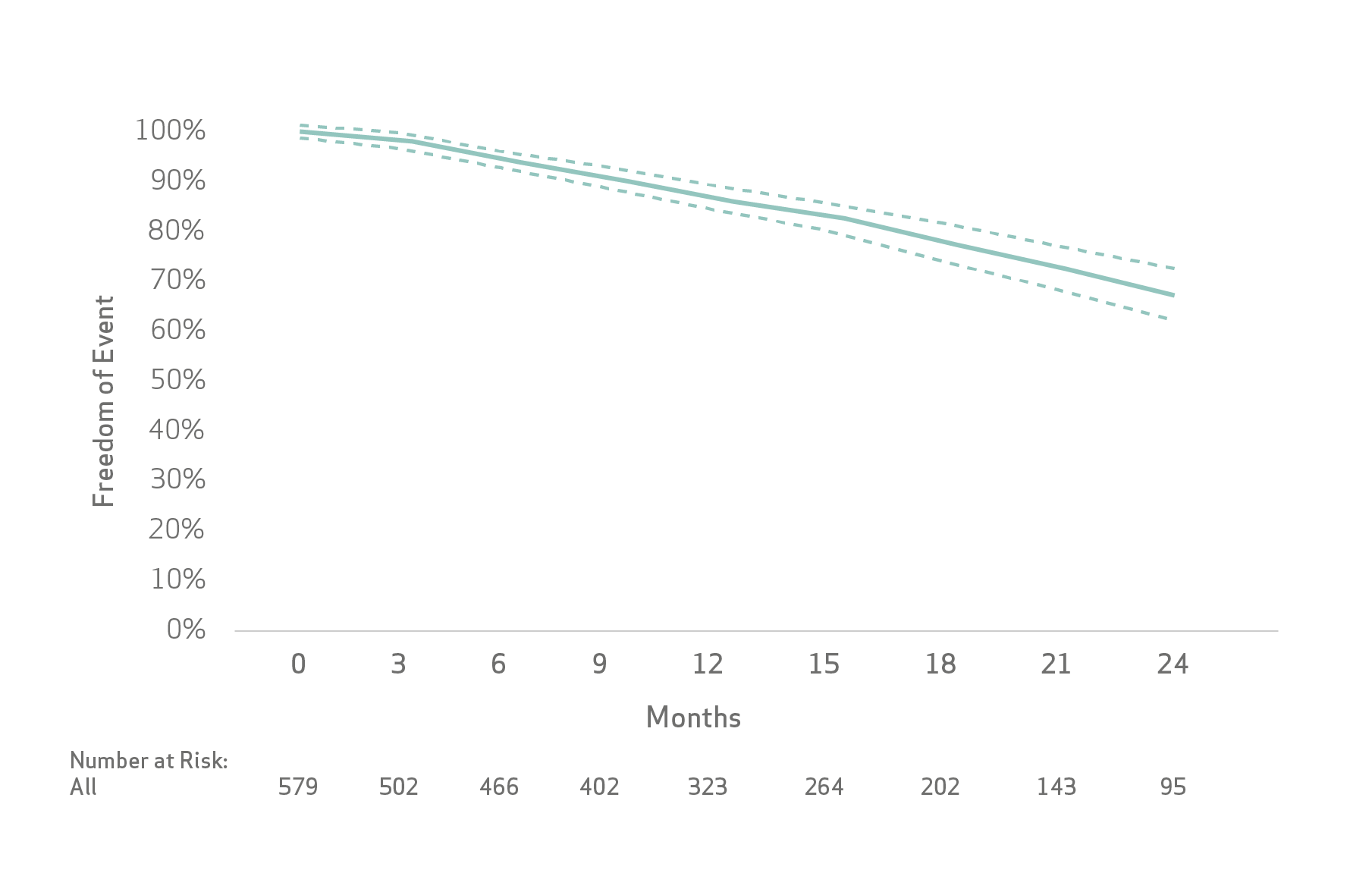
Limitations: As this pooled analysis was not planned prior to conducting the individual studies, analysis limitations are related to differences in the actual recording of the variables, frequency, timing and type of imaging, follow-up period and sample size.
Conclusion
De Baere et al concluded that the treatment of patients with unresectable HCC with LifePearl Microspheres loaded with doxorubicin or idarubicin showed good tolerance with acceptable toxicity and high tumour response rate that translated into promising PFS, TTUP and OS.
Key Takeaway
According to De Baere et al publication, the data from 586 patients treated for unresectable HCC with LifePearl microspheres loaded with doxorubicin or idarubicin showed:
- 57% of treated patients in BCLCC 0/A stage clearly demonstrates treatment stage migration in a real life
- Good tolerance and acceptable toxicity
- High tumor response rate that translated into promising PFS, TTUP and OS
- Overall survival observed in this analysis is similar to the best OS observed in recent TACE trials Waked I. et al. Br J Cancer. 2017; 116(4): 448–454; Golfieri R. et al. Br J Cancer 2014;111:255-64; Kudo M et al. Lancet Gastroenterol Hepatol 2018; 3: 37-46
- Hepatobiliary toxicities as imaging findings were lower than with other DEM-TACE and similar to cTACE studies
Access to the full publication: https://www.annalsofoncology.org/article/S0923-7534(20)39322-4/fulltext
European Economic Area Indications for use
LifePearl™ microspheres are indicated for embolization of blood vessels supplying primary hypervascular tumours or metastases in the liver. Note: LifePearl™ microspheres can be loaded with chemotherapeutic drugs. When used for drug loading, drug loading should be done under a physician's direction, choice and responsibility, based on type and dose of drug most beneficial to the patient. LifePearl™ microspheres are compatible with doxorubicin, epirubicin idarubicin and irinotecan. LifePearl™ microspheres can be drug loaded prior to embolization and then, as a secondary action, elute a local, controlled, and sustained dose to the targeted tumour sites after embolization. LifePearl™ microspheres are not available for sale in all countries. This information is provided only in respect to markets where this product is approved or cleared.
This literature summary is not a systemic review. It is only an example of LifePearl microspheres related literatures.
The use of the LifePearl™ devices in combination with drugs is not cleared or approved in the USA by the Food and Drug Administration. LifePearl™ microspheres are not approved in Canada. Please consult the indication of use with the IFU supplemented with the product.
Please contact your Terumo local sales representative for more information. All brand names are trademarks or registered trademarks of TERUMO CORPORATION and their respective owners. Refer to Instructions for Use for Contraindications, Warnings and Precautions.
©2021 MicroVention Europe CE0297
Manufacturer
MicroVention Europe
30 bis, rue du Vieil Abreuvoir
78100 Saint-Germain-en-Laye - France
Tel: +33(0)1 39 21 77 46
Distributor
Terumo Europe N.V.
Interleuvenlaan 40
3001 Leuven Belgium
Tel: +32 16 38 12 11
