distal Transradial Access (dTRA): A new approach that may further optimize Transradial Access (TRA)
Recently, a new approach for arterial access has been introduced using the distal transradial artery approach (dTRA), which is located at the snuffbox, around the thumb at the dorsal hand.
The distal radial approach is being recommended for the following reasons:
May reduce radial artery occlusion (RAO)1-4
Radiation exposure could decrease
May reduce the rate of complications, (including radial artery spasm)1
Ergonomic positioning may improve
Data from registries and case series are providing insights into the safety and effectiveness of dTRA, but further clinical evidence from large randomized trials will be instrumental to establish the benefits and limitations of this access method and potentially have an important clinical impact for radial operators.
About the DISCO RADIAL trial
The DISCO RADIAL trial is the first large international randomized trial investigating the benefits of dTRA vs conventional TRA.
DISCO RADIAL is a prospective, global, open label, multi-centre randomized controlled trial with plan to include approximately 1,300 patients across 14 sites in 10 countries on who transradial coronary angiography and/or intervention is performed.
The trial compares the novel distal radial access with conventional radial access approach, in terms of radial occlusion rates (RAO) at discharge.
Clinicaltrials.gov. NCT04171570. Available at: https://clinicaltrials.gov/ct2/show/NCT04171570; Terumo data on file.
Learn more about DISCO RADIAL from the Primary Investigators
Dr. Adel Aminian, C.H.U. de Charleroi, Belgium
Dr. Shigeru Saito, Shonan Kamakura General Hospital, Japan
Trial Design
~1,300 patients requiring transradial coronary angiography and/or intervention
RAO at discharge
| Successful sheath insertion rate Access site crossover rate Total procedure time Sheath insertion time Puncture site bleeding according to EASY criteria Overall bleeding according to BARC criteria Vascular access-site complication Rate of radial artery spasm Rate of distal RAO Achievement or not of patent haemostasis by reverse Barbeau test Time required to reach haemostasis Pain associated with the procedure
|
The DISCO RADIAL trial: inclusion and exclusion criteria
Medical condition that may cause noncompliance with the protocol and/or confound data interpretation Chronic hemodialysis ST-elevated myocardial infarction Chronic total occlusion lesions in coronary artery
|
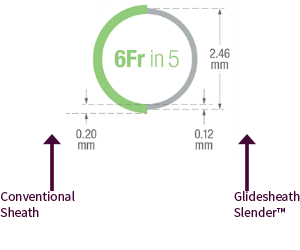
The radial access device used: Glidesheath Slender™
|
![]() 
|
DISCO RADIAL trial results
Results of the DISCO Radial trial are expected later in 2021.
References
1. Coomes E.A. et al. Catheter Cardiovasc Interv 2019; doi:10.1002/ccd.286232. Vefali V. et al. Cardiovasc Revasc Med 2019; doi:10.1016/j.carrev.2019.07.0013. Koutouzis M. et al. Cardiovasc Revasc Med 2019;20:678–80
4. Eid-Lidt G. et al. JACC Cardiovasc Interv 2021; Feb 22;14(4):378-385
