
About MASTER DAPT Study
MASTER DAPT is the largest multi-center
randomized controlled study on the use of short duration of dual
antiplatelet therapy (DAPT) in high bleeding risk patients following
stenting procedures with Ultimaster™/Ultimaster™TanseiTM
drug-eluting bioresorbable polymer stents (DES).
MASTER DAPT study is sponsored by the
European Cardiovascular Research Institute (ECRI, Rotterdam, The
Netherlands) and supported with a restricted research grant by Terumo
Europe. The study is managed by global CROs and data management group
(CERC, Paris, France, Cardialysis, Rotterdam, The Netherlands, CV
quest. Co. Ltd., Tokyo, Japan and CTU, Bern, Switzerland).
MASTER DAPT Study Highlights

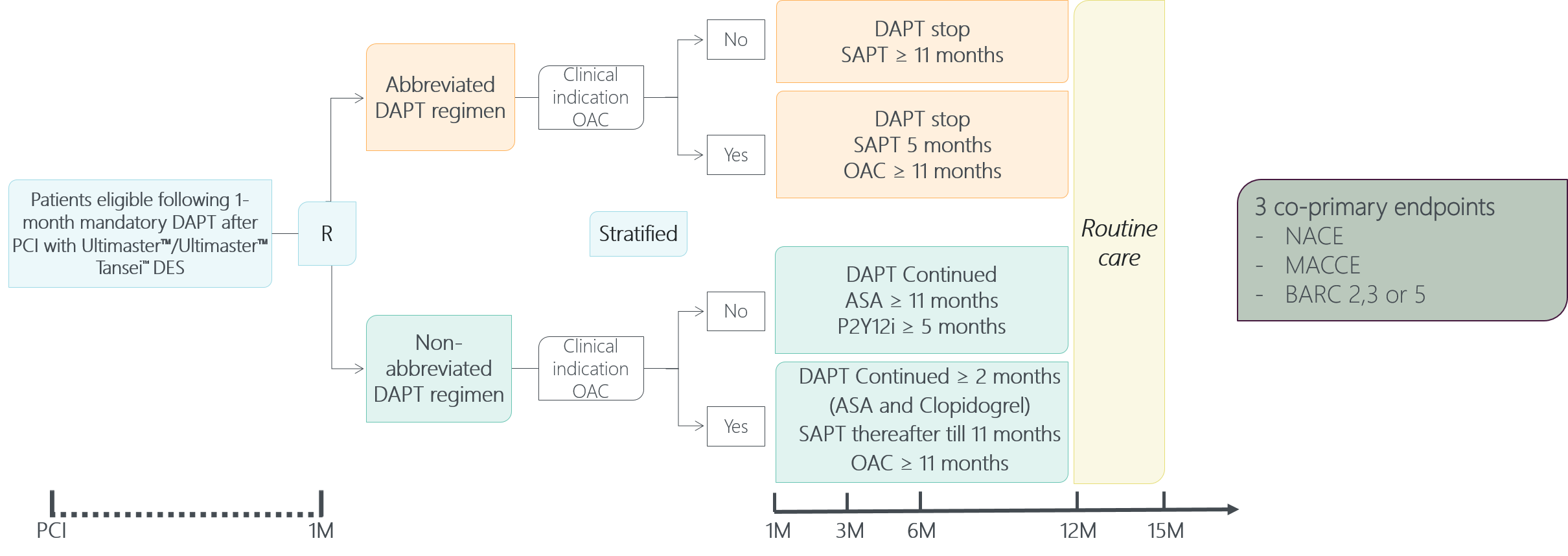
MASTER DAPT Study Design - A true
randomized DAPT study
MASTER DAPT has met the three
coprimary endpoints
1-month
DAPT after implantation of Ultimaster™
family DES does not
increase the ischemic risk, but
reduces the bleeding risk in
high bleeding risk patients
Patients
with acute coronary syndrome, and patients who underwent complex
or multivessel PCI were included
Supports
reduction of DAPT duration to 1 month after
implantation of Ultimaster™ family
DES in a broad cohort of
high-bleeding risk patients
MASTER DAPT
included patients at high bleeding risk who had undergone
implantation of Ultimaster™ family stent; Results may not
extend to patients who are not at high bleeding risk or who receive
other stent types.
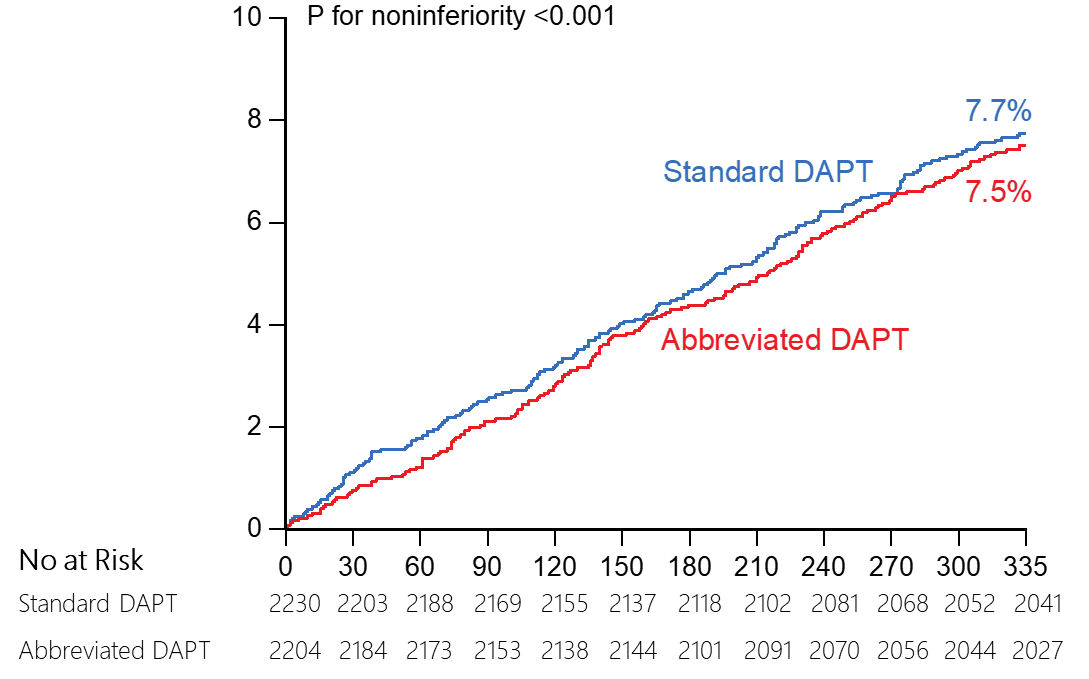
Abbreviated DAPT
non-inferior to standard DAPT in terms of NACE
Net
adverse clinical events (NACE, a composite of death from any cause,
myocardial infarction, stroke, or major bleeding)*
Abbreviated DAPT
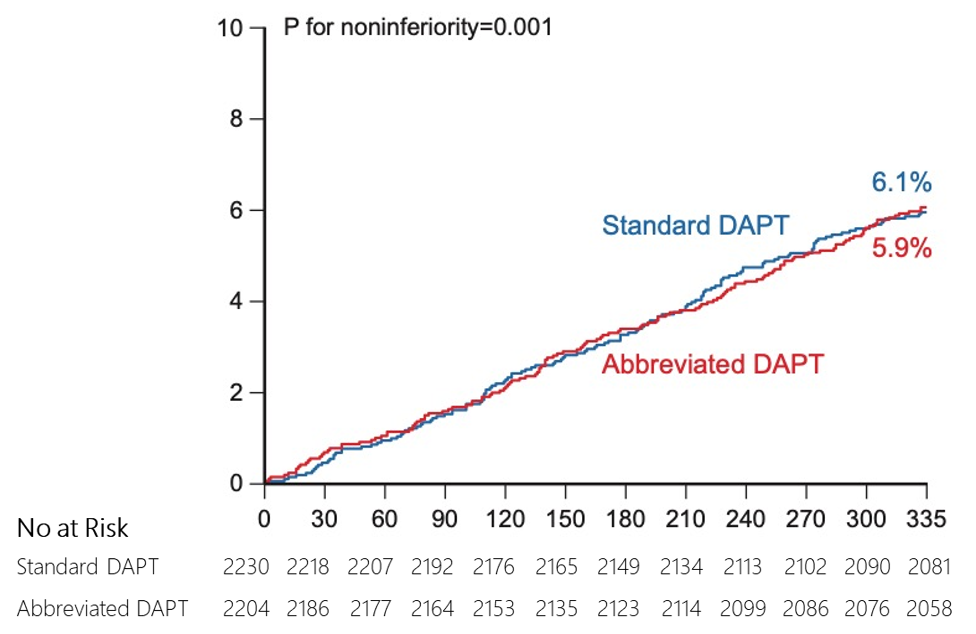
non-inferior to standard DAPT in terms of MACCE
Major adverse
cardiac and cerebral events* (MACCE, a composite of death from any
cause, myocardial infarction, or stroke)
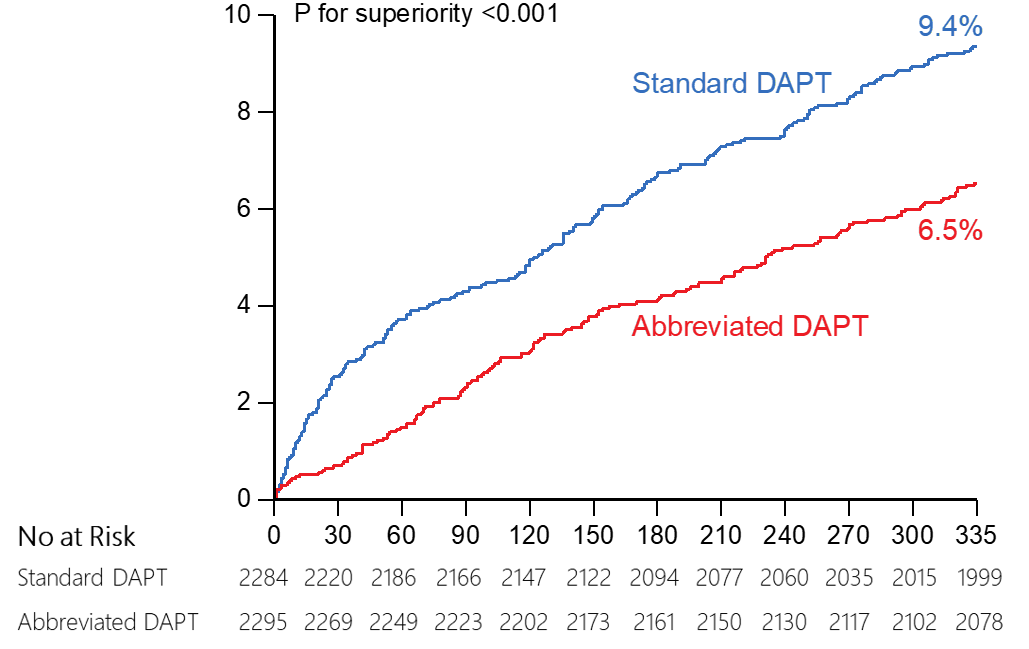
Abbreviated DAPT
superior to standard DAPT in terms of bleeding
Major or clinically relevant non-major
bleeding* (MCB)
Download the Master Dapt Total Population Clinical Summary
Publications on MASTER DAPT
Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. Marco Valgimigli, M.D., Ph.D et al. The New England Journal of Medicine, August 28, 2021, DOI: 10.1056/NEJMoa2108749
Abbreviated
Antiplatelet Therapy in Patients at High Bleeding Risk With or
Without Oral Anticoagulant Therapy After Coronary Stenting: An
Open-Label, Randomized, Controlled Trial. Pieter C. Smits et al.
Circulation, August 29, 2021, 10.1161/CIRCULATIONAHA.121.056680
Abbreviated Antiplatelet Therapy After Coronary Stenting in Patients With Myocardial Infarction at High Bleeding Risk. Smits P, Frigoli E, Vranckx P, et al. J Am Coll Cardiol. 2022 Sep, 80 (13) 1220–1237
Duration of antiplatelet therapy after complex percutaneous coronary intervention in patients at high bleeding risk: a MASTER DAPT trial sub-analysis. Marco Valgimigli, M.D., Ph.D et al. European Heart Journal, September 1, 2022, DOI: 10.1093/eurheartj/ehac284
The Ultimaster Clinical Program – Power of n The UltimasterTM clinical program included more than 50,000 patients covering 16 CE-marked indications. Learn more
