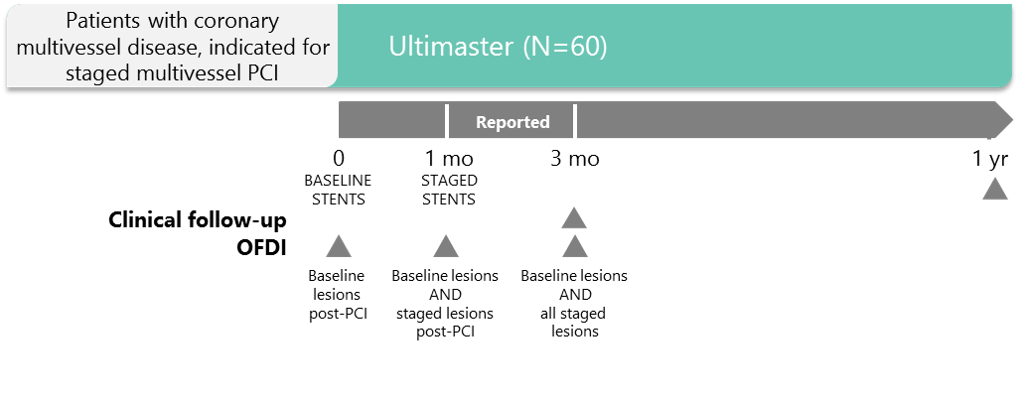
Designed to:
- Assess endothelial coverage at 1, 2 and 3 months by OFDI in patients with multivessel disease scheduled for staged PCI procedure
- Provide a "look" in the artery with OFDI after implantation of Ultimaster
- Investigate possibility for shorter DAPT by generating relevant clinical scientific data
Study Design
- Single arm
- 60 patients with planned staged PCI at 1M/3M
- Total of 6 sites
- 3 countries: France, Germany, Netherlands
- Primary endpoint: OFDI assessed % stent strut coverage at 3M post procedure
Hypothesis: < 20% uncovered struts at 3M post procedure
Primary endpoint was met with strut coverage of 95% achieved by 3 months
- Patient cohort included complex cases and lesions
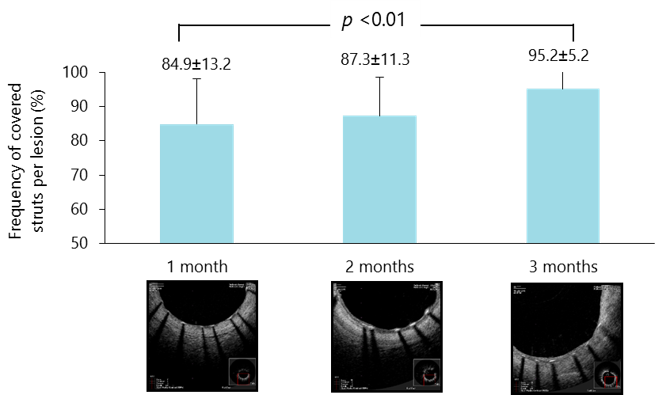
Data are mean±SD
- The mean (SD) number of diseased vessels per patient was 2.2 (0.5), and the mean (SD) number of lesions detected was 3.2 (1.6)
- The mean (SD) total number of lesions treated per patient was 2.1 (0.7)
- Good clinical outcomes were observed between 1 month and 1 year, with no cases of death, MI, TVR, or ST reported
- Incidences of TLR and TLF between 1 month and 1 year were 3.4% for both
- There were no significant differences in baseline patient characteristics, lesion characteristics, or procedural characteristics between the two groups
- 69.8% of procedures that used Ultimaster were performed using transradial access, and the overall procedure success rate was 97.8%
- At 5 years, there was a trend towards better clinical outcomes with Ultimaster than with Xience (statistical significance not met)
- Any death: 9.3% vs 10.8% (p=0.60)
- Any MI: 3.1% vs 5.6% (p=0.19)
- Stent thrombosis: 0.9% vs 1.7% (p=0.43)
References:
MI, myocardial infarction; SD, standard deviation; ST, stent thrombosis; TLF, target lesion failure; TLR, target lesion revascularisation;
TVR, target vessel revascularisation.
MVD, multivessel disease; SD, standard deviation.
Chevalier B et al. Circ Cardiovasc Interv 2017;10. pii: e004801:doi:10.1161/CIRCINTERVENTIONS.116.004801;
Chevalier B et al. Presented at EuroPCR 2017, abstract OP0698;
Iniguez-Romo A et al. Presented at EuroPCR 2018, abstract LBT8727.
Data on file at Terumo Europe.
