First-in-man angiographic and OCT single arm assessment
Prospective trial at 8 sites in Europe:
- Primary endpoint: late loss at 6 months
- Main secondary endpoints: TLF (cardiac death, TV-related MI, clinically indicated TLR), and stent thrombosis
- Angio/IVUS: angiographic in-stent and in-segment binary restenosis rate; in-stent, in-segment, proximal, and distal MLD; IVUS-assessed neointimal hyperplasia volume and percentage volume obstruction; OCT-assessed stent strut coverage and malapposition
Stage: Published and completed
Objectives
- To investigate the 6-month angiographic and long-term clinical outcomes for Ultimaster
- Effect on arterial vessel wall assessed with sophisticated imaging techniques: IVUS and OCT
- Short- and long-term safety and efficacy evaluated by predefined endpoints
- To compare the results for Ultimaster with data from a historical BMS cohort
IVUS, intravascular ultrasound; OCT, optical coherence tomography MI, myocardial infarction; MLD, minimal lumen diameter; TLR, target lesion revascularisation; TV, target vessel.
Barbato E et al. EuroIntervention 2015;11:541–8.
Beleslin B. Presented at EuroPCR 2017, abstract OP0028.
Study design
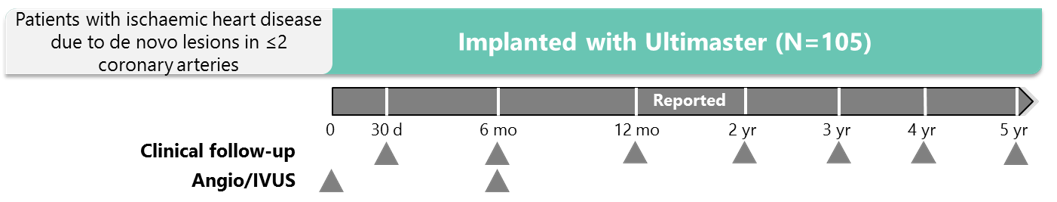
*DAPT duration is counted from the day of the last implanted stent; staging has to be pre-specified at the time of screening and cannot be planned later than
2 months after index PCI. †Patients receiving OAC can stop DAPT 2 months after confirmed randomisation.
ASA, acetylsalicylic acid; DAPT, dual antiplatelet therapy; MACCE, major adverse cardiac and cerebral events; MCB, major or clinically relevant non-major bleeding;
MI, myocardial infarction; NACE, net adverse clinical endpoints; OAC, oral anticoagulant therapy; PCI, percutaneous coronary intervention; SAPT, single antiplatelet therapy.
Terumo. Ultimaster Drug Eluting Stent Instructions for Use, version 0.1-2018; MASTER DAPT trial. Available at https://clinicaltrials.gov/ct2/show/NCT03023020?term=master+dapt&rank=1 (accessed April 2018).
Primary endpoint: clinically significant and superior efficacy at 6 months with Ultimaster vs a historical BMS groups
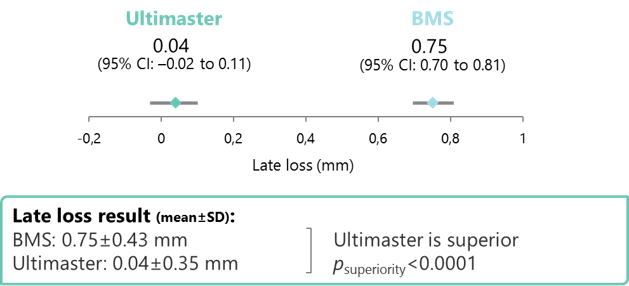
CI, confidence interval.
Kaname used as the BMS platform.
Hypothesis: based on estimated Kaname late loss: 0.90±0.50 mm, late loss of 0.40 mm (0.90–0.50 mm) is considered upper limit for
CENTURY study.
Barbato E et al. EuroIntervention 2015;11:541–8.
A high degree of stent coverage was achieved at 6 months with Ultimaster
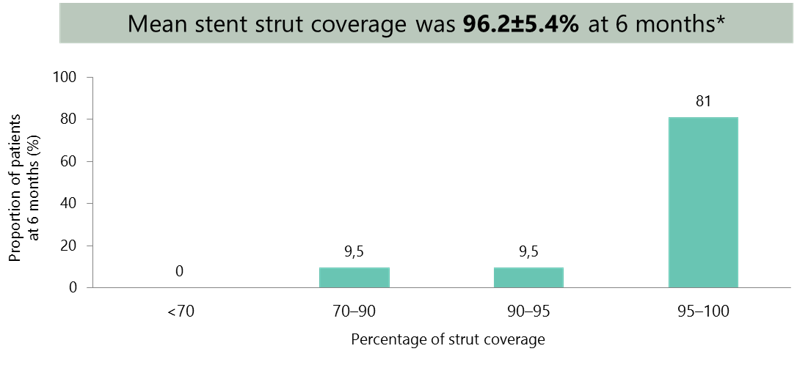
*Mean±SD.
Barbato E et al. EuroIntervention 2015;11:541–8;
Barbato E. Presented at EuroPCR 2015, abstract OP047
Low rates of stent-related events with Ultimaster up to 5 years
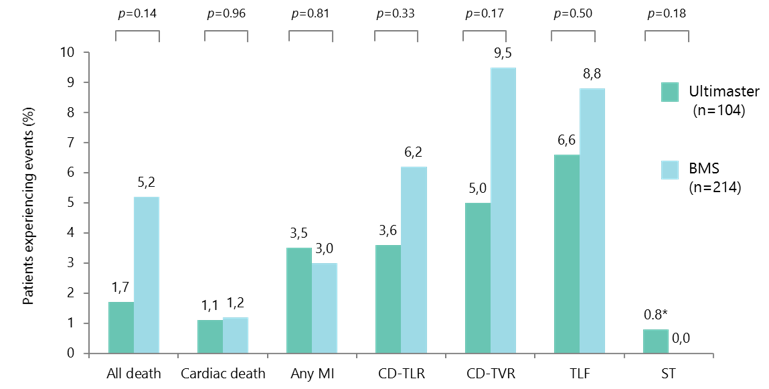
Ultimaster vs BMS, propensity matched analysis; Kaname used as the BMS platform.
*One acute stent thrombosis due to untreated dissection.
CD-TLR, clinically driven target lesion revascularisation; CD-TVR, clinically driven target vessel revascularisation; ST, stent thrombosis.
Beleslin B. Presented at EuroPCR 2017, abstract OP0028.
Similar rates of TVF* at 5 years for both stents
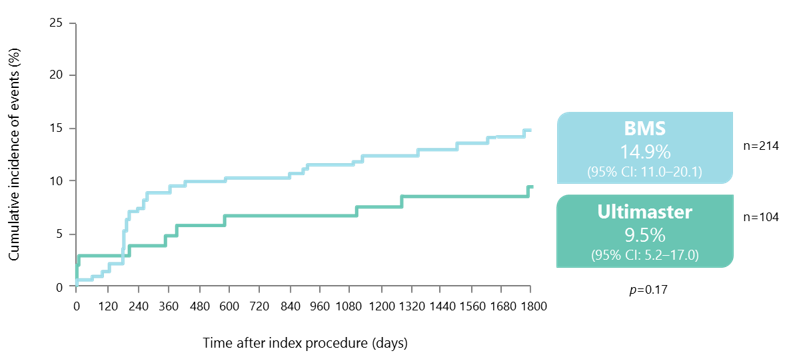
*Composite of cardiac death, target vessel-related MI and CD-TVR.
Kaname used as the BMS platform.
Beleslin B. Presented at EuroPCR 2017, abstract OP0028.
- Ultimaster was superior to the BMS withrespect to in-stent late loss: achieving a 95% late loss reduction
- IVUS and OCT data support the efficacy and safety profile of Ultimaster:
- Thin and homogeneous layer of neointima covered stent struts 6 months after implantation
- Average strut coverage rate of 96.2%
- Rates of clinical events remained low with Ultimaster up to 5 years after implantation, with a low TLR rate, and just one acute ST reported due to untreated dissection
Barbato E. Presented at EuroPCR 2015, abstract OP047; Barbato E et al. EuroIntervention 2015;11:541–8; Beleslin B. Presented at EuroPCR 2017, abstract OP0028.
