Background:
Currently, only two recent retrospective studies have evaluated B-TACE using DEMs (B-DEM-TACE) in HCC and showed positive results in terms of safety and efficacy. We report here the first prospective study assessing the feasibility and safety of B-TACE using PEG embolizing microspheres loaded with 75 mg doxorubicin (B-DEM-TACE) in a selected population of HCC patients.
Objective:
Evaluation of safety and efficacy of selective balloon-occluded transarterial chemoembolization using polyethylene glycol embolizing microspheres in patients with hepatocellular carcinoma.
Methods
24 patients with 40 HCC lesions underwent B-DEM-TACE
Adverse events were evaluated at 24 h and 1 month
Imaging response according to modified response evaluation criteria in solid tumours was assessed at 1, 3 and 6 months
Results
Gontran Verset et al registry demonstrated:
Safety:
Nine patients (37.5%) received a second treatment and two of them (22.2%) went on to a third treatment without worsening of the liver function.
All 24 patients were candidates for multimodal treatment after B-DEM-TACE due to the lack of hepatic injury.
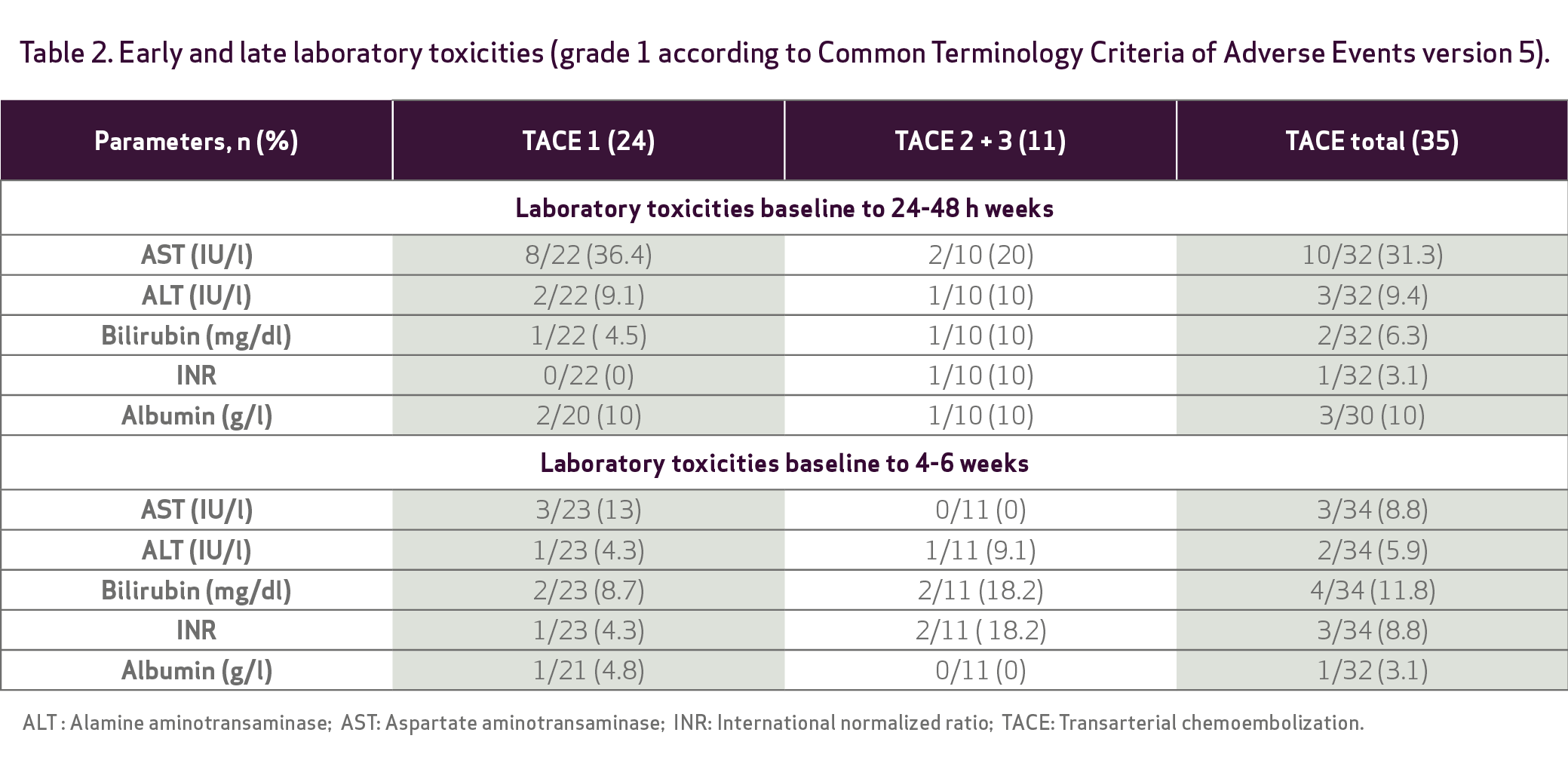
Efficacy:
Conclusion
Gontran Verset et al concluded that B-DEM-TACE has proven to be an effective treatment for HCC patients with a very good safety profile. In our series we have observed fewer AEs than previously described with other catheters, suggesting an added value of this technique in terms of liver function preservation. If future trials will confirm these observations, B-DEM-TACE may become a valuable tool for intra-arterial LRTs mostly for frail patients.
Key Takeaway:
The main findings of Gontran Verset et al are that:
This technique enables to improve the selectivity of TACE, which is an added value for the treatment of HCC particularly in cirrhotic patients, who are most likely to present an impaired liver function.
Currently, a key point of locoregional therapy is the preservation of liver function, ever since the development of various systemic therapies that improve OS of these patients.
Access to full publication: https://www.futuremedicine.com/doi/10.2217/hep-2020-0022
Use/Indications
European Economic Area Indications for use of Occlusafe™ Temporary Occlusion Balloon Catheter
- Occlusafe™ is intended to be used for gaining temporary occlusion in the peripheral vasculature. The Occlusafe™ provides temporary vascular occlusion which is useful in selectively stopping or controlling blood flow
- Occlusafe™ is not available for sale in all countries. This information is provided only in respect to markets where this product is approved or cleared
- Occlusafe™ is manufactured by Terumo Clinical Supply Co., Ltd. and exclusively distributed by Terumo Europe NV in the EMEA region. All brand names are trademarks or registered trademarks of TERUMO CORPORATION and their respective owners. Scientific and clinical data related to this document are on file at Terumo Clinical Supply Co., Ltd. Refer to Instructions for Use for additional information.
This literature summary is not a systemic review. It is only an example of Occlusafe™Temporary Occlusion Balloon Catheter related literatures.
Please contact your Terumo local sales representative for more information. All brand names are trademarks or registered trademarks of TERUMO CORPORATION and their respective owners. Refer to Instructions for Use for Contraindications, Warnings and Precautions.
©2021 TERUMO CLINICAL SUPPLY CO, LTD. CE0344
Manufacturer
TERUMO CLINICAL SUPPLY Co., Ltd.
3 Kawashima-Takehayamachi
Kakamigahara Gifu, Japan 501-6024
Tel: +81(0)3-6742-8770
Distributor
Terumo Europe N.V.
Interleuvenlaan 40
3001 Leuven Belgium
Tel: +32 16 38 12 11
