Angio-Seal™ VIP is
- a
bioabsorbable implantable medical device1
- intended for use in
closing and reducing the time to hemostasis of common femoral arterial punctures following arterial access procedures1
- proven to be
safe, effective, and support rapid closure of the femoral access site2
ANGIO-SEAL™ VIP DEMONSTRATED HIGHER DEPLOYMENT SUCCESS RATES VS OTHER VCD
- 100% deployment success rate with Angio-Seal™ VIP 6Fr for diagnostic procedures
- 98.5% deployment success rate with Angio-Seal™ VIP 6Fr for interventional procedures
- Angio-Seal™ VIP has demonstrated higher deployment success rate compared to other VCD5
ANGIO-SEAL™ VIP DEMONSTRATED LOWER COMPLICATION RATES VS MANUAL COMPRESSION
- Angio-Seal™ VIP provides safe, effective, and rapid closure of the femoral access site2
- Angio-Seal™ VIP has been clinically proven to have significantly lower complication rates than Manual Compression after PCI6
ANGIO-SEAL™ VIP REDUCES THE TIME TO HEMOSTASIS
ANGIO-SEAL™ VIP REDUCES THE TIME TO AMBULATION
- Angio-Seal™ VIP is indicated for use to allow patients to ambulate safely as soon as possible after sheath removal and device placement1
- Angio-Seal™ VIP has clinically proven to reduce time to ambulation7
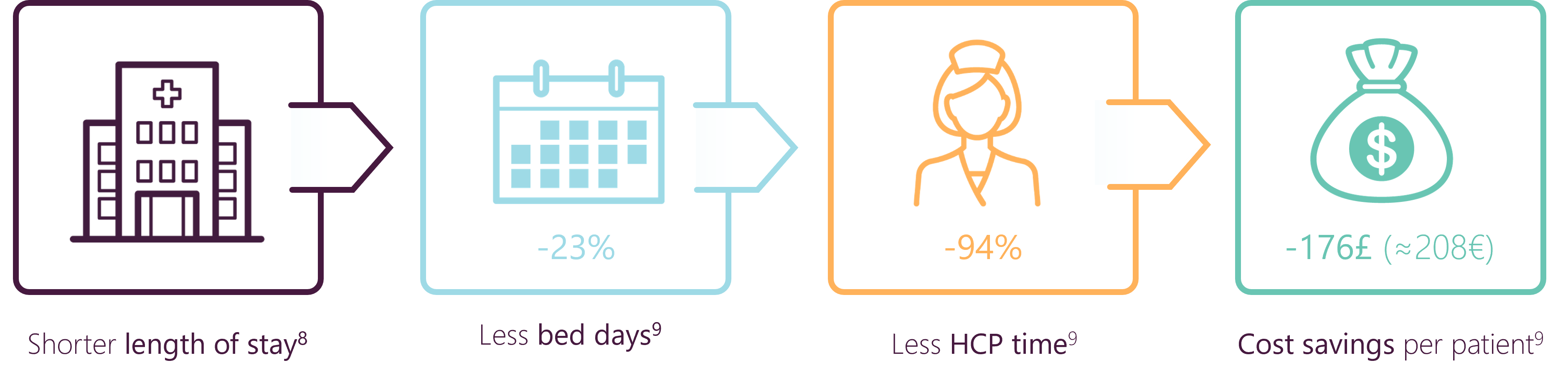
ANGIO-SEAL™ VIP SUPPORTS EARLY DISCHARGE & COST SAVING
- Angio-Seal™ VIP is associated with a significantly shorter length of hospital stay compared to manual compression8
- Compared to manual compression, Angio-Seal™ VIP could lead to cost savings9 after endovascular procedures, despite the additional costs of the VCD itself
ANGIO-SEAL™ VIP IS SAFE TO USE IN ANTEGRADE AND RETROGRADE FEMORAL PUNCTURE10
ANGIO-SEAL™ VIP & ARTERIAL RESTICK
.png)
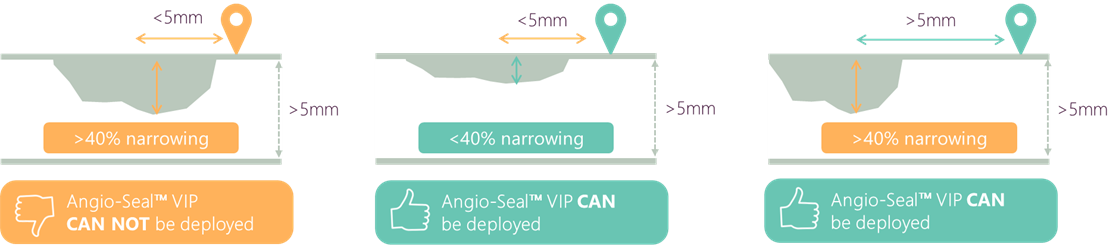
ANGIO-SEAL™ VIP & PATIENTS WITH PERIPHERAL VASCULAR DISEASE1
- in patients with arteries >5 mm diameter
- where there is found to be no luminal narrowing of 40% or greater within 5 mm of the puncture site
References
-
Angio-Seal™ VIP Instruction for use.
-
Wong H.F. et al. Am J Neuroradiol. 2013; 34: 397-401.
-
Martin J.L. et al. Catheter Cardiovasc Interv. 2008 Jan 1;71(1):1-5.
-
Modi S. et al. Indian J Radiol Imaging. 2013;23(2):134-138.
-
Jones L.E. et al. Ann Vasc Surg. 2018 Aug;51:10-17.
-
Kreutz R.P. et al J Soc Cardiovasc Angiogr Interv. 2022 Jun 29;1(5).
-
Alshehri A.M. et al. Int J Angiol. 2015 Jun; 24(2): 133–136.
-
Veasey R.A. et al. International Journal of Clinical Practice 2008, 62(6), 912–918.
-
Goueffic Y. et al. Ann Vasc Surg. 2023 Jul;93:64-70
-
Chaudhuri A. et al. Eur J Vasc Endovasc Surg. 2014 Aug;48(2):220-5.
-
Ipema J. et al. CRIT LIMB ISCHEM 2023 March 2.
-
Tellez A.et al. EuroIntervention. 2010 Jan;5(6):731-6.
