Bleeding is a frequent complication in cardiac surgery procedures, especially following aortic interventions and can cause prolonged operating times, increased blood loss and higher risk of postoperative morbidity and mortality. 1,2,3
Effective hemostasis is therefore of utmost importance and "the key to the success of aortic surgery" as stated by Morita and coworkers it his recent study published in 20194.
Surgical sealants have been developed and are used in addition to the traditional methods like sutures and staples to prevent bleeding and achieve complete hemostasis. However, the conditions during aortic surgery are challenging for the application of sealants as the wet tissue surface, the movement of the aorta and the high blood pressure in the vessel can prevent efficient sealing.
AQUABRID® is a new surgical sealant especially developed for aortic procedures. It reacts with water (blood) and forms an elastic layer that adheres tightly to the tissue. It is safe and effective in achieving hemostasis, even when blood coagulation is inhibited during cardiac surgery.5,6,7
The product was introduced in Japan by Terumo Corporation under the brand name Hydrofit® in 2014 and has rapidly gained market share due to its unique properties. AQUABRID® is now commercially available in the EU and registrations for further countries in Eastern Europe, Middle East and Africa are ongoing. First clinical uses of AQUABRID® have taken place in Germany and Turkey with excellent outcomes.
"We are excited to have this innovative new product as an addition to our surgical product portfolio", says Sebastien Van den Berghe, Vice President Terumo Cardiovascular EMEA. "AQUABRID® opens a new market for us in a segment of growing importance for our business", he concluded.
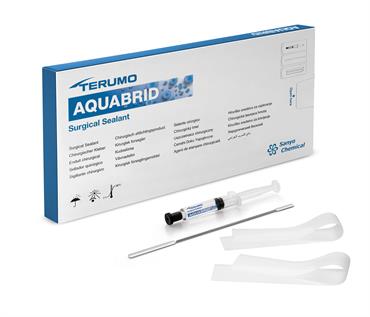
About the product
AQUABRID® is a fully synthetic surgical sealant developed for aortic surgical procedures. When in contact with water, it reacts and forms a strong, elastic layer within three to five minutes. This makes it ideal for use in wet conditions — AQUABRID® can be applied directly to wet natural and/or artificial tissue (e.g., an aortic graft).5,8
AQUABRID® comes with a syringe filled with the gel like sealant, a spatula for even distribution and two silicone sheets that help transfer the sealant to the desired application side and holding it there during the time of reaction.
The sealant is stored at room temperature (1-30°C) and does not need any form of preparation. Just take of the protective cap and AQUABRID® can be applied.9
Material: Fluorinated urethane polyether prepolymer9
Indication: AQUABRID® is indicated for use as an adjunct to standard methods of cardiovascular surgical repair to seal (such as sutures, staples, electrocautery, and/or patches) related to aorta surgery. Indicated sites are sutured sites and anastomosis of aorta (associated dissection, rupture or aneurysm).9
AQUABRID® is a product developed and manufactured by Sanyo Chemical Industries, Ltd.
- End -
About Terumo Cardiovascular Group
Terumo Cardiovascular Group manufactures and markets medical devices for cardiac and vascular surgery with an emphasis on cardiopulmonary bypass, intra-operative monitoring and vascular grafting. The company is headquartered in Ann Arbor, Michigan, with manufacturing operations in the U.S., Europe and Asia. It is one of several subsidiaries of Terumo Corporation of Japan that is focused exclusively on cardiac and vascular specialties. www.terumo-cvgroup.com
About Terumo Europe
Terumo Europe is a core player in the EMEA healthcare market by providing best in class quality medical products and services. We are a strong actor of the Terumo Group by contributing to innovation and sustainable growth. Terumo Europe produces, distributes, markets and sells a vast range of medical devices in five main business units: Interventional Systems, Pharmaceutical Solutions, Medical Products, Cardiovascular Products and Diabetes Management. Terumo Europe EMEA headquarters and production facilities are located in Leuven (Belgium), production facility in Knowsley (UK), European Distribution Center in Genk (Belgium) and sales offices across EMEA. www.terumo-europe.com
About Terumo Corporation
Terumo (TSE:4543) is a global leader in medical technology and has been committed to "Contributing to Society through Healthcare" for nearly 100 years. Based in Tokyo and operating globally, Terumo employs more than 25,000 associates worldwide, to provide innovative medical solutions in more than 160 countries and regions. The company started as a Japanese thermometer manufacturer, and has been bolstering healthcare ever since. Now, its extensive business portfolio ranges from vascular intervention and cardio-surgical solutions, blood transfusion and cell therapy technology, to medical products essential for daily clinical practice. Terumo will further strive to be of value to patients, medical professionals, and society at large.
About Sanyo Chemical
Sanyo Chemical is a manufactures and sales of performance chemicals, established in 1949 in Kyoto. Performance chemicals featured by functional characteristics respond various needs such as more beautiful, safer, more comfortable and environment friendlier. Sanyo Chemical started as a manufacturer of soap and textile agents, and diversified our product line to satisfy the needs of the market. Sanyo Chemical company operates in six business segments: toiletries & health, petroleum & automotive, plastic, textiles, info/electronic & construction/others. Their solutions are essential to our daily lives from automotive to daily electronics, passing by cosmetics and medical equipment. Now Sanyo Chemical have over 3,000 different types of products and an international presence. Sanyo Chemical endeavor to create better life and society through performance chemicals in various fields.
Hydrofit® is a registered trademark of Sanyo Chemical Industries, Ltd. In Japan.
AQUABRID® is a registered trademark of Sanyo Chemical Industries, Ltd.
1 Dickneite et. al. 2000: Prevention of Suture Hole Bleeding Using Fibrin Sealant: Benefits of Factor XIII; JSR 93(2):201-205. p.201.
2 Lodi et.al. 2012: Management of haemostasis in surgery: sealant and glue applications; Lippincott Williams & Wilkins p.1.
3 Rogers et.al. 2016: Meta‐analysis of the use of surgical sealants for suture‐hole bleeding in arterial anastomoses; Br J Surg. 103(13):1758-1767. p.1758.
4 Morita et.al. 2019: Randomized clinical trial of an elastomeric sealant for hemostasis in thoracic aortic surgery; General Thoracic and Cardiovascular Surgery, p.1.
5 Eto et al. 2007: Elastomeric surgical sealant for hemostasis of cardiovascular anastomosis under full heparinization. Europ. J. Cardio Surg. 2007; 730-734.
6 Sales data Terumo Japan.
7 Morita et.al. 2019: Randomized clinical trial of an elastomeric sealant for hemostasis in thoracic aortic surgery; General Thoracic and Cardiovascular Surgery, p.1.
8 Oda et.al. 2009: Follow-up papers: Experimental use of an elastomeric surgical sealant for arterial hemostasis and its long-term tissue response; ICVTS 10:258-261, p.258.
9 AQUABRID® Instruction for use.
