About the Investigator-Initiated Studies Program
Terumo established an internal cross-functional review process to assess the requests for support of investigator-initiated studies. A timely and complete submission will be reviewed by an internal scientific committee and Grants & Donations committee.
Terumo may support external clinical investigations with researchers who can demonstrate clear evidence of high ethical and scientific standards that govern clinical research in human subjects as stipulated by the International Council for Harmonisation (ICH) E6(R2) Guideline for Good Clinical Practice (GCP).
Investigator-Initiated Studies Program
Investigator-initiated studies have scientific and medical merit and are developed and sponsored by an independent researcher. An IIS is conducted independently without Terumo participation or involvement. The IIS researcher may request Terumo to provide any or all of the following: funding, medical device(s), laboratory test(s)/procedure(s), equipment and training.
Responsibilities of the independent researcher:
- Study design and conduct
- Protocol review and approval
- Obtaining the ethics committee and/or regulatory authority approvals, as required
- Collecting the informed consents
- Data analysis and interpretation
- Publication
- Compliance with local laws, regulations and guidelines
- Reporting to regulatory authorities
Other responsibilities include:
- Meeting specific milestones
- Submitting updates to Terumo Medical Affairs personnel
- Authoring final study report
Areas of Interest
The Holmium Platform (QuiremScout® microspheres, QuiremSpheres® microspheres, Q-Suite™ software)
- Demonstrate the value of holmium-SIRT for the treatment of primary liver cancer or liver metastases secondary to colorectal cancer (mCRC) or neuroendocrine tumors (mNET):
- Safety and efficacy of Holmium-166 SIRT (either as stand-alone or in combination) in selected study population
- Studies designed for understanding the role and demonstrating the value of Holmium-SIRT as an immune system modulator
- Comparative studies in a well-defined setting
- Real-World performance of holmium-SIRT, including data on long-term safety and side effects
- Quality-of-Life and cost effectiveness studies
- Use and value of the complete suite of solutions of the Holmium platform in every potential indication in terms of improved:
- Patient selection
- Treatment planning (e.g. personalized activity calculation)
- Treatment and treatment evaluation
IIS Proposal Process

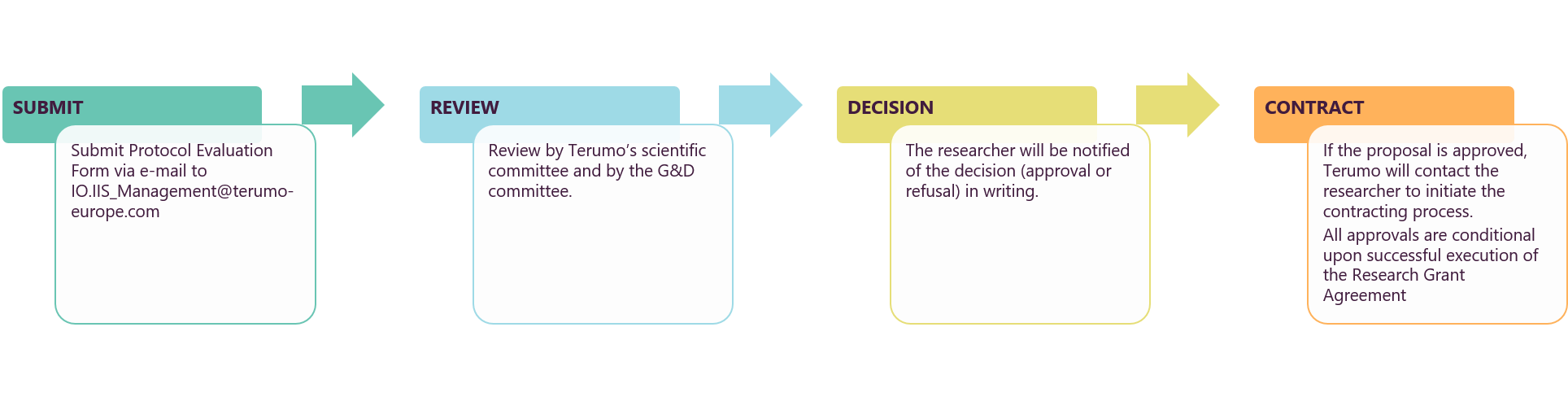
How to Submit
The independent researcher submits a fully completed and signed Protocol Evaluation Form via email to
IO.IIS_Management@terumo-europe.com
Download
