Background
The advantages of chemoembolization (TACE) in mCRC are several, such as reducing drug leakage, liver and systemic toxicity. TACE is widely used for patients with colorectal cancer liver metastases (CRC-LM) after failure of surgery or systemic chemotherapy and can be used for both pre- and post-operative downsizing, reducing the time to surgery, and prolonging overall survival.
Objective
To study the safety profile and efficacy of polyethylene glycol embolics loaded with irinotecan for the treatment CRC-LM.
Methods
Patients with CRC-LM, refractory to systemic chemotherapy with liver involvement < 50%, treated with LifePearlTM Microspheres, loaded with 100mg irinotecan.
Tumor response evaluated by RECIST1.1, and quality of life (QoL) was monitored and evaluated with the Palliative Performance Scale (PPS).
Results
Fiorentini et al study (n=50) demonstrated:
- Tumor Response
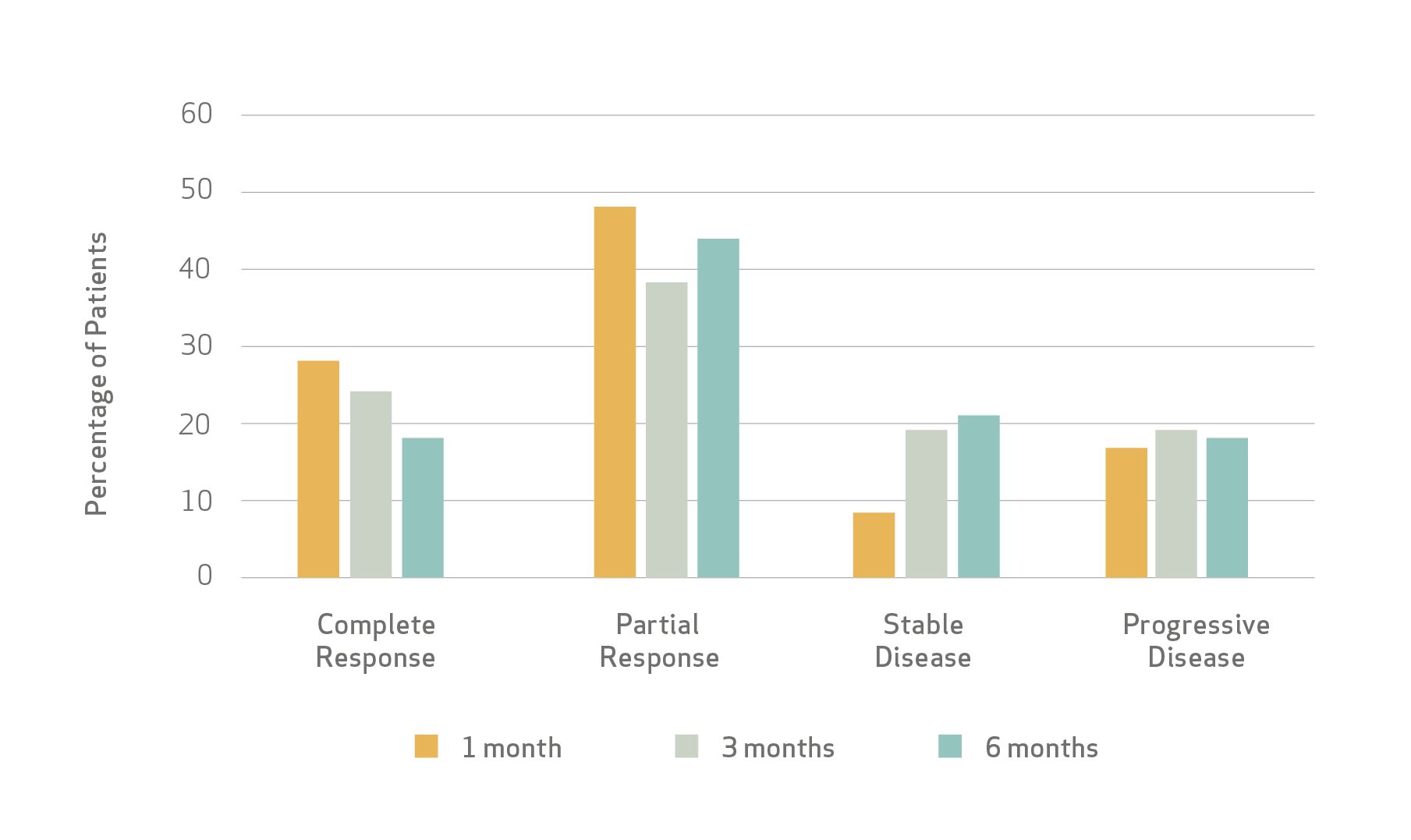
Median time to progression for patients who progressed was 2.5 months.
Safety profile:
TACE procedures were performed with no complications. Observed side effects (mild or moderate intensity) were: Pain in 32% of patients, increase of transaminase levels in 20% and fever in 14%, whereas 30% of patients did not complain any adverse event.
QoL a 90% of PPS at each time point after TACE suggesting good physical and social functions, and health perception
The study has several limitations, such as the small number of patients observed and the short time of follow-up.
Conclusion
Fiorentini et al concluded that the therapy of unresectable CRC-LM with LifePearl microspheres loaded with irinotecan was effective with high tumor response and limited toxicity providing good QoL showing non-inferior results than previous drug eluting beads.
Key Takeaway
According to Fiorentini et al, the treatment of unresectable CRC-LM with trans-arterial treatment using LifePearl microspheres loaded with irinotecan was effective with good QoL and safety profile.
Access to full publication: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5605338/
European Economic Area Indications for use
LifePearl™ microspheres are indicated for embolization of blood vessels supplying primary hypervascular tumours or metastases in the liver. Note: LifePearl™ microspheres can be loaded with chemotherapeutic drugs. When used for drug loading, drug loading should be done under a physician's direction, choice and responsibility, based on type and dose of drug most beneficial to the patient. LifePearl™ microspheres are compatible with doxorubicin, epirubicin idarubicin and irinotecan. LifePearl™ microspheres can be drug loaded prior to embolization and then, as a secondary action, elute a local, controlled, and sustained dose to the targeted tumour sites after embolization. LifePearl™ microspheres are not available for sale in all countries. This information is provided only in respect to markets where this product is approved or cleared.
This literature summary is not a systemic review. It is only an example of LifePearl microspheres related literatures.
The use of the LifePearl™ devices in combination with drugs is not cleared or approved in the USA by the Food and Drug Administration. LifePearl™ microspheres are not approved in Canada. Please consult the indication of use with the IFU supplemented with the product
Please contact your Terumo local sales representative for more information. All brand names are trademarks or registered trademarks of TERUMO CORPORATION and their respective owners. Refer to Instructions for Use for Contraindications, Warnings and Precautions.
©2021 MicroVention Europe CE0297
Manufacturer
MicroVention Europe
30 bis, rue du Vieil Abreuvoir
78100 Saint-Germain-en-Laye
France
Tel: +33(0)1 39 21 77 46
Distributor
Terumo Europe N.V.
Interleuvenlaan 40
3001 Leuven Belgium
Tel: +32 16 38 12
