Background
Despite the clearly defined technical specifications for Drug eluting Microspheres (DEM) for transcatheter arterial chemoembolization (TACE), no standard administration protocol was designated among different sites regarding diameter of the used particles.
Objective
To report clinical effectiveness and toxicity profile, of combined 100 μm ± 25 and 200 μm ± 50 epirubicin-loaded PEG LifePearl™ microspheres drug-eluting embolization microsphere in patients with hepatocellular carcinoma (HCC).
Methods
Embolization was initiated with 100 μm ± 25 microspheres, and if stasis was not achieved, 200 μm ± 50 microspheres were administered.
Each syringe (2 mL) of PEG microsphere was loaded with 50 mg of epirubicin.
Results were evaluated using mRECIST at 1, 3–6, 9–12, and 15–18 months.
Toxicity profile was assessed by laboratory testing before and after the procedure.
Postembolization syndrome (PES) was defined as onset of fever/nausea/pain after the procedure.
Results
Lucatelli et al study (n=36) demonstrated:
36 patients (mean age 69.9 y ± 10.8; 26 men, 10 women; 54 naïve lesions) were treated with18 months of follow-up,
In 10 of 21 lesions, < 2 cm in diameter (47.5%) stasis was achieved with 100 μm ± 25 microspheres only, whereas all other lesions required adjunctive treatment with 200 μm ± 50 microspheres.
Efficacy:
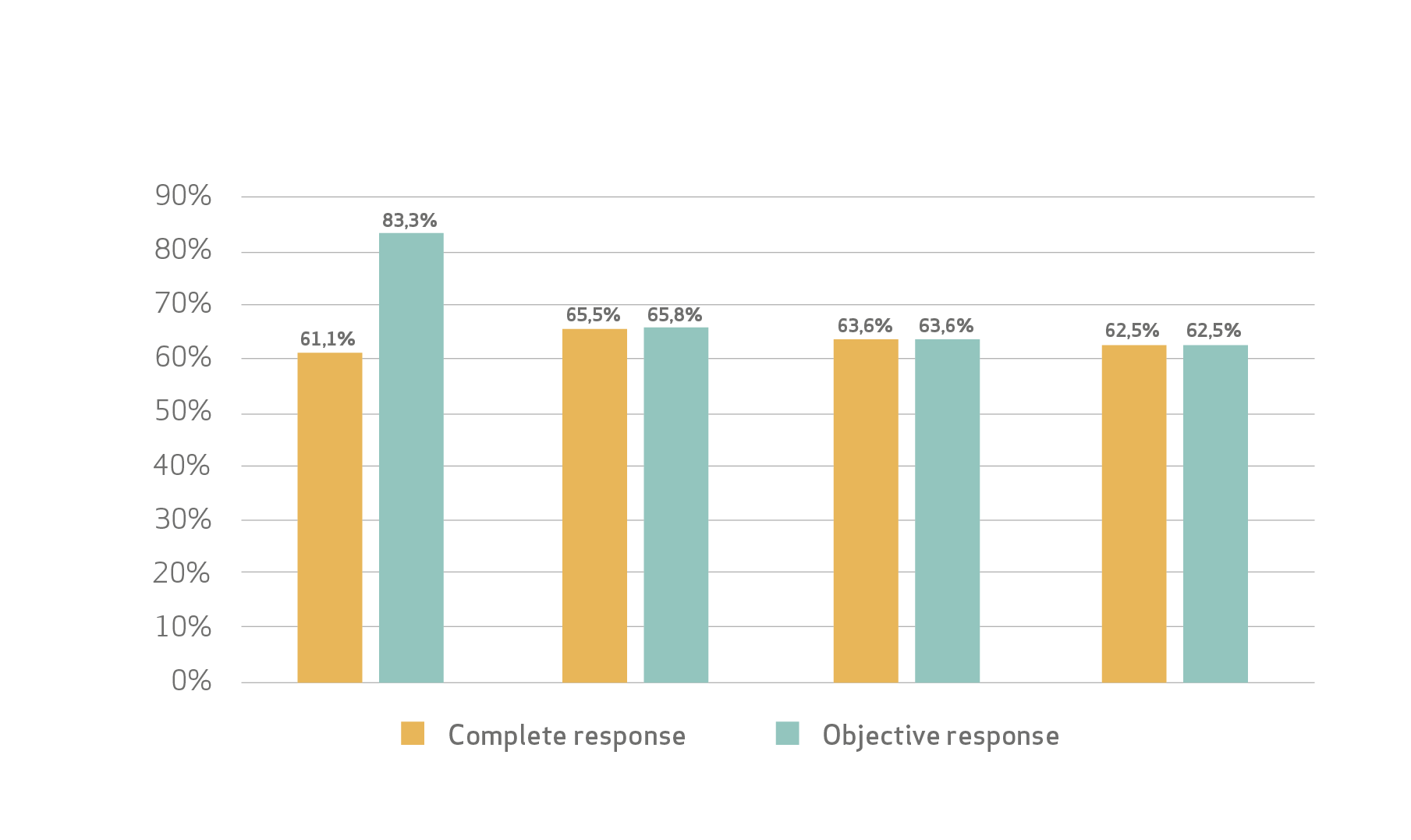
Safety profile:
Limitations:
This study has several limitations. First, the enrolled sample size (36 patients; 54 treated tumors) is small. Second, it was a single-arm experience without control for other embolization protocols, in particular, with regard to the microsphere diameter or to the drug used. Third, despite having strictly standardized the embolization protocol, the amount of microsphere and drug administered to each lesion remains unpredictable, so a proportion of patients received only the 100 μm ± 25 microspheres.
Conclusion
Lucatelli et al concluded that the proposed combined embolization protocol consisting of sequential administration of 100 μm ± 25 and 200 μm ± 50 LifePearl microspheres preloaded with epirubicin showed sustained CR and DC rates of 60% and 90%, respectively, up to 15–18 months from the initial treatment.
Key Takeaway
According to Lucatelli et al, the combined Drug eluting embolization microsphere sizing strategy (100 + 200 μm), when LifePearl microspheres are loaded with epirubicin was technically feasible and yielded promising results in terms of effectiveness and toxicity.
Access to the full publication: https://pubmed.ncbi.nlm.nih.gov/30713031/
European Economic Area Indications for use
LifePearl™ microspheres are indicated for embolization of blood vessels supplying primary hypervascular tumours or metastases in the liver. Note: LifePearl™ microspheres can be loaded with chemotherapeutic drugs. When used for drug loading, drug loading should be done under a physician's direction, choice and responsibility, based on type and dose of drug most beneficial to the patient. LifePearl™ microspheres are compatible with doxorubicin, epirubicin idarubicin and irinotecan. LifePearl™ microspheres can be drug loaded prior to embolization and then, as a secondary action, elute a local, controlled, and sustained dose to the targeted tumour sites after embolization. LifePearl™ microspheres are not available for sale in all countries. This information is provided only in respect to markets where this product is approved or cleared.
This literature summary is not a systemic review. It is only an example of LifePearl microspheres related literatures.
The use of the LifePearl™ devices in combination with drugs is not cleared or approved in the USA by the Food and Drug Administration. LifePearl™ microspheres are not approved in Canada. Please consult the indication of use with the IFU supplemented with the product.
Please contact your Terumo local sales representative for more information. All brand names are trademarks or registered trademarks of TERUMO CORPORATION and their respective owners. Refer to Instructions for Use for Contraindications, Warnings and Precautions
©2021 MicroVention Europe CE0297
Manufacturer
MicroVention Europe
30 bis, rue du Vieil Abreuvoir
78100 Saint-Germain-en-Laye - France - Tel: +33(0)1 39 21 77 46
Distributor
Terumo Europe N.V.
Interleuvenlaan 40 - 3001 Leuven Belgium - Tel: +32 16 38 12 11
