Background
Common femoral artery (CFA) access is still the leading approach for peripheral endovascular interventions of lower extremity arterial disease (LEAD), despite the development of radial artery access for coronary endovascular procedures.
For sheaths ≤8F, two groups of Vascular Closure Devices (VCDs) are described according to their mechanism of action: The polymer-based VCDs such as FemoSeal™ Vascular Closure System* (Terumo) and the suture-based VCDs like Perclose ProGlide™ Suture-mediated Closure System (Abbott).
The presence of rigid calcified structure or osteoid metaplasia in the CFA could jeopardize the successful deployment of suture-based VCDs which may not able to go through the calcifications. In this case polymer based VCD might be more efficient.
VCDs differ in term of secondary hemostasis, as bail-out technique. For ProGlide™ the guide wire is left in place and a second device can be used, whereas in the case of FemoSeal™ it is retrieved before implantation and no additional VCD can be deployed.
Primary objective
To compare the efficacy of the polymer-based VCD (FemoSeal™) and the suture-based VCD (ProGlide™) in achieving hemostasis at the femoral access site after lower limb arterial endovascular revascularization.
Methodology
- Study design:
- Investigator-initiated trial
- Multicenter (7 French sites) funded by the French Ministry of Health
- Prospective
- Randomized: Intention-to-treat statistical analysis (ITT)
- Single blind
- Population: 230 patients
- Enrollment: December 2017-April 2019
- Procedure type: endovascular diagnostic or treatment in LEAD
- Access: Duplex scan-guided Retrograde puncture/ 5-7F introducers
- Study devices: FemoSeal™ or ProGlide™
- Follow-up (FU): 5 hours, 1 day, 30 days (duplex scan)
- Procedures were performed by trained vascular interventionalists certified in the use of both devices by their respective manufacturers
Primary outcome
Technical success defined
as full & permanent hemostasis evaluated just after
and 5 h following the VCD application (patients’ ambulation ability for
100 m) without need in additional VCD, Manual Compression (MC) or other
site intervention, without
a 2-g/dL reduction in haemoglobin.
Secondary outcomes
i.) major and minor complications rate
ii.) time to discharge
iii.) quality of life assessment
iv.) total cost of VCDs per treated leg evaluated at the same purchase price
Results for primary outcome
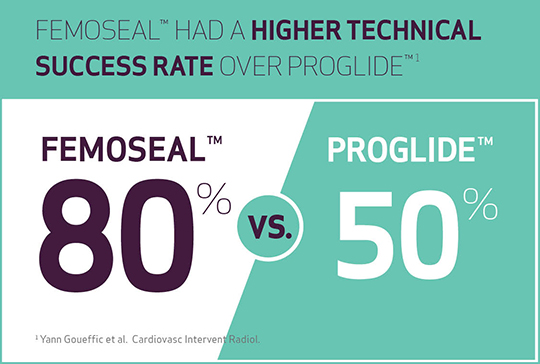
- The technical success rate was higher with FemoSeal™ vs ProGlide™ (p<0.0001)
- 80% with FemoSeal™
- 50% with ProGlide™
After sensitivity analysis (excluding patients that underwent MC) superiority of FemoSeal™ in term of technical success was confirmed (FemoSeal™ patients: 88.99% vs ProGlide™ patients: 62.39% (p<0.0001)).
Results for secondary outcomes
- With ProGlide™: 23 additional VCDs used vs none with FemoSeal™
- Need in MC: 45 patients with Proglide™ vs 19 patients with FemoSeal™
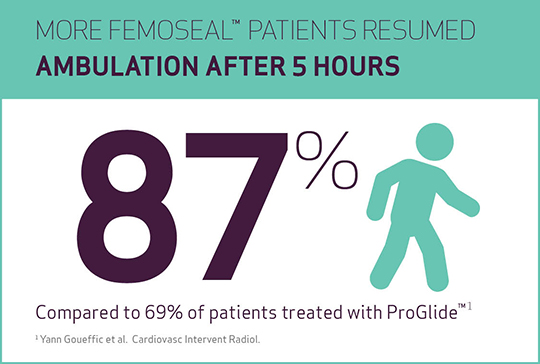
- Resumption of ambulation (walk distance of 100 m after 5 h) was significantly shorter in FemoSeal™ vs ProGlide™, respectively 87% vs 69% of cases (p=0.001).
- Similar complications rate, with FemoSeal™ & ProGlide™
- At one month, there was no significant difference between the two groups in terms of symptomatology and quality of life.
Conclusions
- FemoSeal™ shows a better technical success than ProGlide™ for femoral arterial closure in patients having arterial imaging or endovascular treatment for PAD.
- FemoSeal™ could be preferred over ProGlide™ to optimize the efficiency of the ambulatory management/same-day discharge of patients.
Access the full publication:
*FemoSeal™ Vascular Closure System is indicated for use in closing the femoral arterial puncture (arteriotomy) in patients who have undergone percutaneous catheterization using a 7F (2.33 mm) or smaller procedural sheath.
