Terumo Europe NV announced today that the Iberis™ system was featured at the AIM Radial course held Sep 26-27, 2013 in New York. Dr. Jean Fajadet and Dr. Benjamin Honton, Clinique Pasteur, performed Transradial Renal Nerve Ablation using the Iberis™ system. The procedure was transmitted live from Toulouse, France during the AIM Radial scientific session.
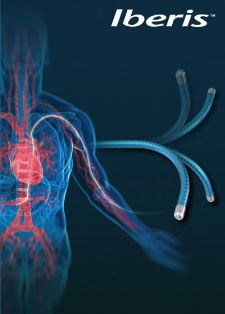
Renal Nerve Ablations are tipically performed via femoral artery access. Terumo and AngioCare Medical Co., Ltd. developed the specially designed Iberis™ System which facilitates radial access to the renal artery for a less invasive treatment.
"Radial access is a safe and feasible way for renal artery catheterisation and represents a favourable approach for Sympathetic Renal Denervation" said Dr. Benjamin Honton, France
"Radial access allows us to treat Resistant Hypertension patients who were difficult to treat via conventional Femoral access" said Dr. Jean Fajadet, France
About Iberis™ system
Renal nerve ablation using a catheter based technique is an effective treatment option for the large population of hypertension patients who do not respond to conventional drug therapy.
The Iberis™ system, which granted CE certification in April 2013, is a catheter delivering high radio frequency energy through the wall of renal artery to ablate or disrupt the surrounding renal sympathetic nerves. The Iberis™ system is available in a design for femoral access as well as the minimally invasive design for radial access.
For more information, please visit
http://www.terumo-europe.com/cardiology/products/iberis-renal-sympathetic-denervation-system.php
Contact : Mr. Olivier Archer, Terumo Europe Interventional Systems
Iberis™ is a trademark of Terumo (China) Holding Co., Ltd. And ANGIOCARE MEDICAL CO., Ltd.
Iberis™ is manufactured by ANGIOCARE MEDICAL CO., Ltd. and exclusively distributed in Europe by Terumo Europe NV
