Objectives
- Assess the pharmacokinetics of sirolimus after Ultimaster implantation
- Evaluate the systemic concentration and potential toxicity of sirolimus
- Assess endothelial function
- Investigate the safety and tolerability profile of Ultimaster, along with therapeutic outcomes
Stojkovic S et al. Fundam Clin Pharmacol 2014;29:95–105.
Study design
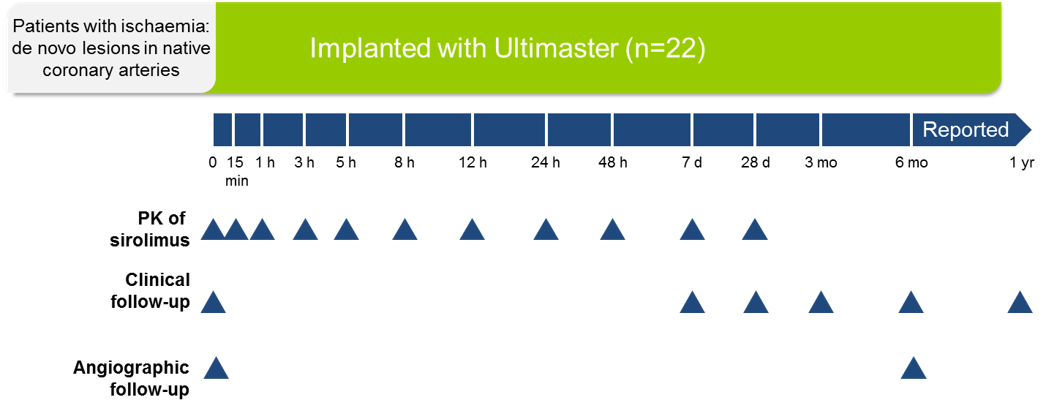
PK, pharmacokinetics.
Stojkovic S et al. Fundam Clin Pharmacol 2014;29:95–105; Data on file at Terumo Europe
Other findings
- 6 months:
- Endothelial function well preserved
- In-stent late loss: 0.10±0.28 mm
- Up to 12 months:
- No signs of sirolimus toxicity or other safety concerns
Stojkovic S et al. Fundam Clin Pharmacol 2014;29:95–105.
