Designed to:
- confirm safety and efficacy of Ultimaster drug eluting stent used in STEMI patients by proving superiority in efficacy and non-inferiority in safety versus bare metal stent
- generate further evidence for benefits of primary PCI with DES in patients with STEMI
- generate further evidence for benefits of bioresorbable polymer
Study Design
- randomized (3:1) Ultimaster vs Kaname, single blind
- 500 patients with acute STEMI
- 16 sites
- 5 countries
- Triple primary endpoint at 1 (safety), 6 (efficacy) and 12 months (safety/efficacy)
- Secondary endpoints: clinical, procedural, device related, angiographic...
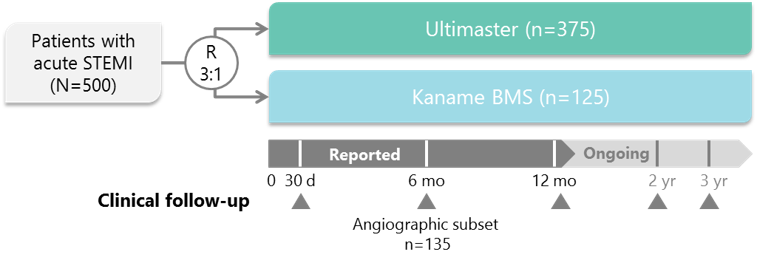
BMS, bare-metal stent; MI, myocardial infarction; R, randomisation; ST, stent thrombosis; STEMI, ST-segment elevation myocardial infarction;
TV, target vessel; TVF, target vessel failure; TVR, target vessel revascularisation.
Valdes-Chavarri M. Presented at EuroPCR 2016.
Triple primary endpoint
composite of all-cause death, recurrent MI, unplanned infarct related artery revascularization, stroke,
definite ST, and major bleeding
In-stent late loss
Efficacy and safety (12 months):
TVF and a composite of cardiac death, recurrent TV MI, TVR
MI, myocardial infarction; ST, stent thrombosis; TV, target vessel; TVF, target vessel failure; TVR, target vessel revascularisation.
Valdes-Chavarri M. Presented at EuroPCR 2016.
STEMI: clinical evidence from MASTER
- In general, there were no significant differences in baseline patient characteristics, lesion characteristics, or procedural characteristics between the two groups, with the following exceptions:
- The number of stents per lesion was significantly higher with Ultimaster than with Kaname (1.5 vs 1.3; p=0.04)
- Total stent length per patient was significantly longer with Ultimaster than with Kaname (29.7 mm vs 26.1 mm; p=0.01)
- At 3 years, clinical outcomes were significantly better with Ultimaster than with Kaname for all clinically driven revascularisation, CD-TLR, and CD-TVR
CD-TLR, clinically driven target lesion revascularisation; CD-TVR, clinically driven target vessel revascularisation; STEMI, ST-segment elevation myocardial infarction.
Valdes-Chavarri et al. Eurointervention 2018; doi: 10.4244/EIJ-D-17-01087. Stankovic G et al. Presented at EuroPCR 2018, abstract LBT8754.
MASTER (STEMI): clinical outcomes at 1 year
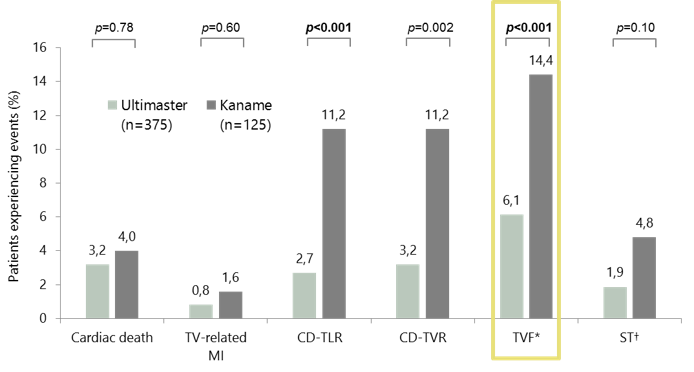
*Composite endpoint of cardiac death and MI not clearly attributable to a non-TV and CD-TVR.
†Definite or probable ST according to ARC.
N numbers are the number of patients at baseline in the per-protocol analysis: 98.4% follow-up achieved at 12 months.
ARC, Academic Research Consortium; CD-TLR, clinically driven target lesion revascularisation; CD-TVR, clinically driven target vessel revascularisation;
MI, myocardial infarction; ST, stent thrombosis; STEMI, ST-segment elevation myocardial infarction; TV, target vessel; TVF, target vessel failure.
Valdes-Chavarri et al. Eurointervention 2018; doi: 10.4244/EIJ-D-17-01087
MASTER (STEMI): clinical outcomes at 3 years
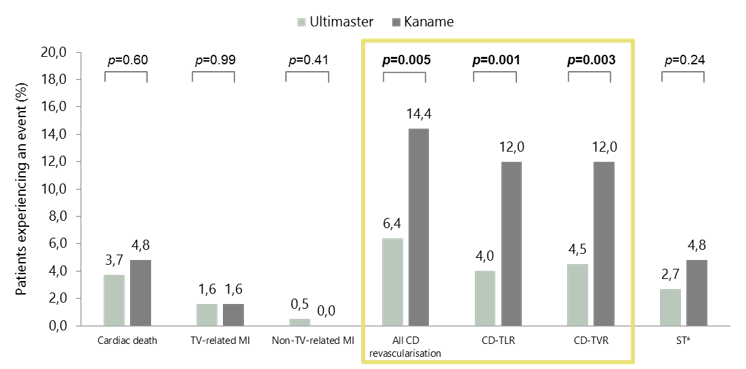
*Definite or probable ST.
CD, clinically driven; CD-TLR, clinically driven target lesion revascularisation; CD-TVR, clinically driven target vessel revascularisation;
MI, myocardial infarction; ST, stent thrombosis; STEMI, ST-segment elevation myocardial infarction; TV, target vessel.
Stankovic G et al. Presented at EuroPCR 2018, abstract LBT8754.
