Multivessel disease
- The
revascularization strategy (complete versus incomplete revascularization) in
multivessel disease patients treated with Ultimaster
DES was assessed at 1-year follow-up
- Complete
revascularization was associated with better relief from angina, lower
mortality, less target vessel failure (TVF), and lower patient oriented
composite endpoint (POCE)
- Ultimaster
DES demonstrated excellent results in this complex population
e-ULTIMASTER MVD subgroup: Clinical outcomes at 1 year
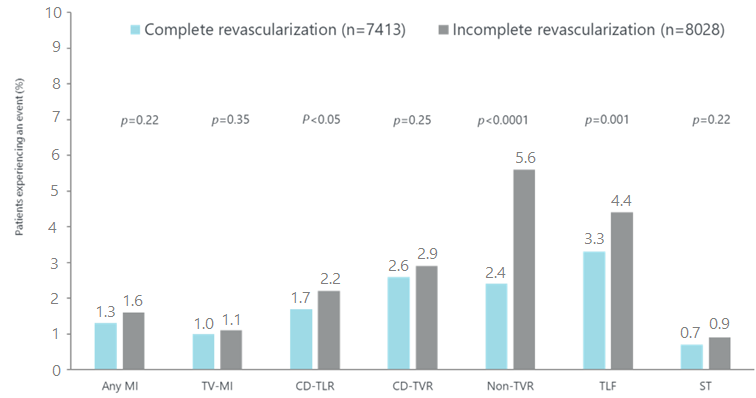
CD-TLR: clinically-driven target
lesion revascularization; CD-TVR: clinically-driven target vessel
revascularization; MI, myocardial infarction; MVD, multivessel disease; ST,
definite/probable stent thrombosis; TLF, target lesion failure (composite endpoint
of cardiac death, target vessel-related myocardial infarction, and clinically
driven target lesion revascularization); TLR, target lesion revascularisation;
; TV,
target vessel; TV-MI: target vessel myocardial infarction.
Williams T et al. Catheter Cardiovasc Interv 2022;99:961-967
High risk ACS
-
The 1-year results of 14,874 STEMI or NSTEMI patients treated with Ultimaster stent showed a higher risk of cardiac death and stent thrombosis in this high hisk ACS group
-
However, the clinical outcomes in both high risk ACS and the rest of the patient group were very good
e-ULTIMASTER High risk ACS subgroup: Clinical outcomes at 1 year
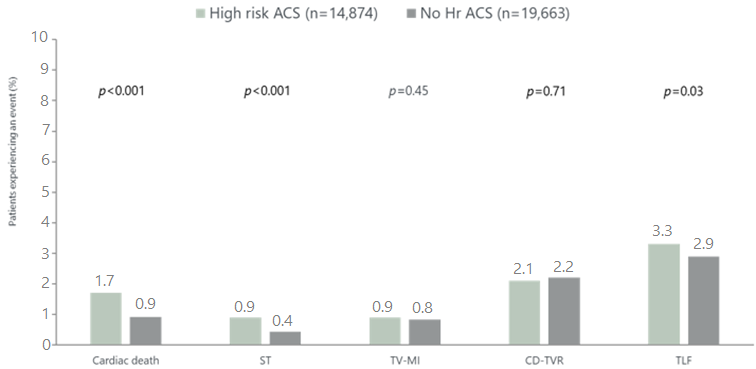
ACS, acute coronary syndrome; CD-TVR, clinically driven target vessel revascularisation; Hr, high risk; MI, myocardial infarction; ST, definite/probable stent thrombosis; TLF, target lesion failure (composite endpoint of cardiac death, target vessel-related myocardial infarction, and clinically driven target lesion revascularization); TV, target vessel
Laanmets P. PCR e-course 2020
CTO
- 1722 patients were treated for CTO with mean age (SD) of 63.8 (10.7) years
- Patients in the CTO group had a higher prevalence of comorbidities
- At 1 year, favourable clinical outcomes were observed with Ultimaster, with low incidences of cardiac death, TV-related MI, CD-TLR, and stent thrombosis
e-ULTIMASTER CTO subgroup: Clinical outcomes at 1 year
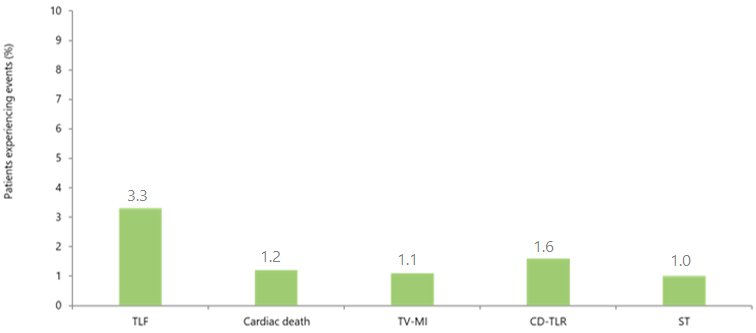
|
|
CTO subgroup n=1722
CD-TLR, clinically driven target lesion revascularisation; CTO, chronic total occlusion; MI, myocardial infarction; ST, definite /probable stent thrombosis; TLF, target lesion failure (a composite of cardiac death, target vessel myocardial infarction and clinically driven target lesion revascularization); TV, target vessel;
Rumoroso J. PCR e-course 2020
