Background:
Effectiveness of the initial TACE procedure is reduced in challenging HCC tumours, and these tumours frequently require retreatment.
Objective:
The aim of this retrospective multicentric study was to compare the tumour response rates of Balloon-occluded Transarterial Chemoembolisation (B-TACE) to non-B-TACE using propensity score matching (PSM) in patients with hepatocellular carcinoma and to investigate the clinical benefit, such as lower rates of TACE re-intervention achieved using B-TACE.
Methods
- Retrospective, real-life registry in 6 European centres of 530 patients affected by early or intermediate stage HCC not amenable to curative treatment
- 96 patients were treated with B-TACE using Occlusafe™ Temporary Occlusion Balloon Catheter with either Lipiodol-based cTACE or DEM-TACE
- 434 patients were treated with non-B-TACE.
- Objective responses (OR) and complete response (CR) rates after the first session and the number of TACE reinterventions were evaluated using PSM (91 patients per arm).
- Patients underwent imaging assessment CT or MRI at 1, 3 and 6 months after TACE
Results
Golfieri et al registry demonstrated:
Safety:
- No intraprocedural complications
- Severe adverse events were rare and were similar between the B-TACE and the non-B-TACE arms, with a significant prevalence of medically controlled pain and nausea in the B-TACE patients.
- Post-embolization syndrome (PES) rates were 8.8% in non-B-TACE and 41.8% in B-TACE (p<0.001), with no significant differences between groups regarding major adverse events.
Efficacy:
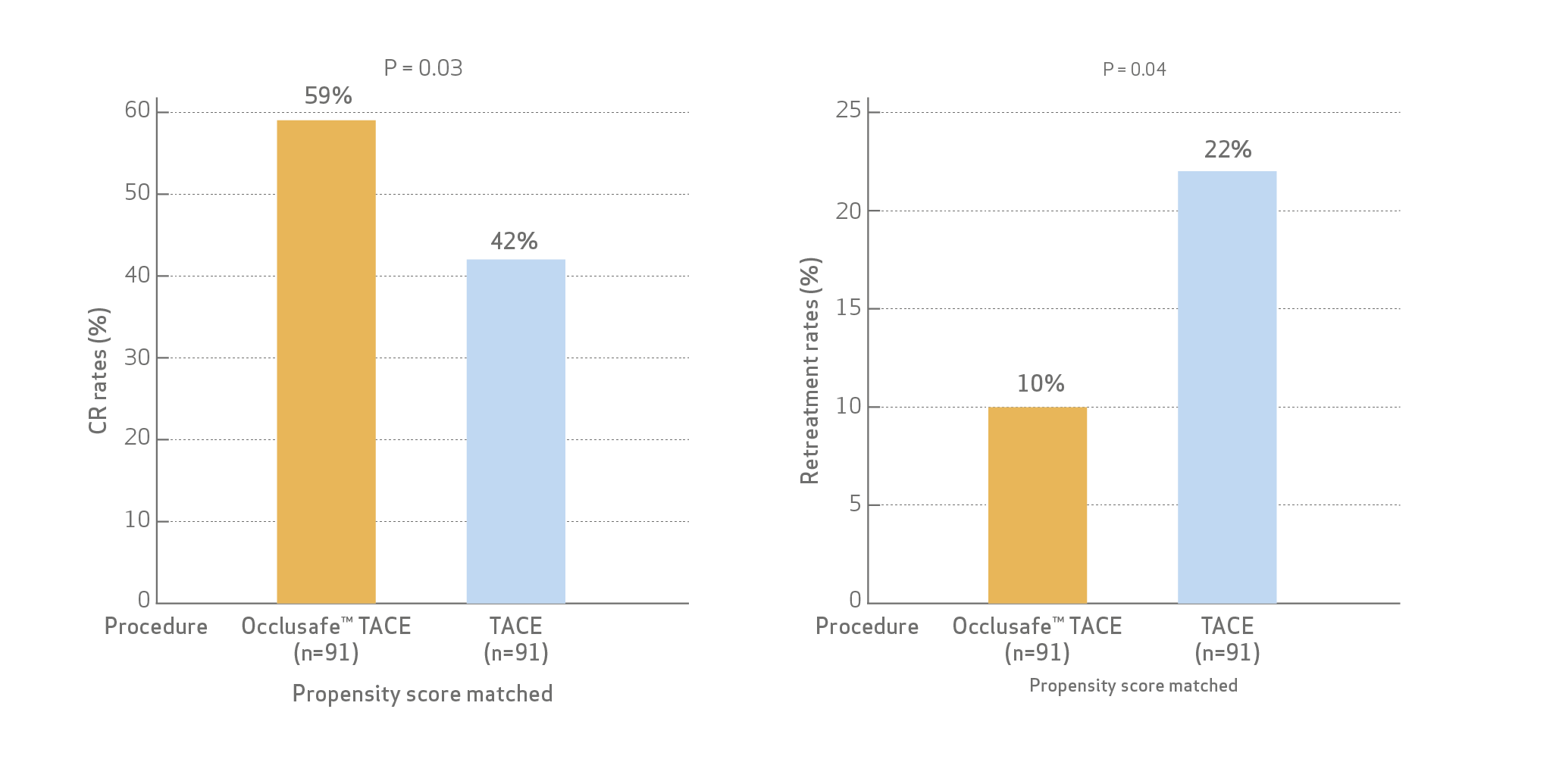
- The best target OR after PSM were similar for both B-TACE and non-B-TACE (90.1% and 86.8%, p = 0.644)
- CR at 1–6 months was significantly higher for B-TACE (59.3% vs. 41.8%, p = 0.026).
- Patients treated with B-TACE had a significantly lower retreatment rate during the first 6 months as compared to patients undergoing cTACE/DEM-TACE (9.9% vs. 22.0%, p = 0.041).
Conclusion
Rita Golfieri et al concluded that B-TACE with Occlusafe™ is safe and effective, achieving higher CR rates for treating HCCs when compared to non-B-TACE. Patients undergoing B-TACE have a significantly lower retreatment rate within the first 6 months, but higher PES rates. A higher CR rate allows for better tumour control and possible prolonged survival.
Key Takeaway:
The main findings of Rita Golfieri et al are that:
- B-TACE with Occlusafe™ provides that CR at 1–6 months was significantly higher vs non B-TACE
- Patients treated with B-TACE had a significantly lower retreatment rate during the first 6 months
Access to full publication: https://link.springer.com/article/10.1007/s00270-021-02805-5
Use/Indications
European Economic Area Indications for use
- Occlusafe™ is intended to be used for gaining temporary occlusion in the peripheral vasculature. The Occlusafe™ provides temporary vascular occlusion which is useful in selectively stopping or controlling blood flow
- Occlusafe™ is not available for sale in all countries. This information is provided only in respect to markets where this product is approved or cleared
- Occlusafe™ is manufactured by Terumo Clinical Supply Co., Ltd. and exclusively distributed by Terumo Europe NV in the EMEA region. All brand names are trademarks or registered trademarks of TERUMO CORPORATION and their respective owners. Scientific and clinical data related to this document are on file at Terumo Clinical Supply Co., Ltd. Refer to Instructions for Use for additional information.
This literature summary is not a systemic review. It is only an example of Occlusafe™ Temporary Occlusion Balloon Catheter related literatures.
Please contact your Terumo local sales representative for more information. All brand names are trademarks or registered trademarks of TERUMO CORPORATION and their respective owners. Refer to Instructions for Use for Contraindications, Warnings and Precautions.
©2021 TERUMO CLINICAL SUPPLY CO., LTD. CE0344
Manufacturer
TERUMO CLINICAL SUPPLY CO., LTD.
3 Kawashima-Takehayamachi
Kakamigahara Gifu, Japan 501-6024
Tel: +81(0)3-6742-8770
Distributor
Terumo Europe N.V.
Interleuvenlaan 40
3001 Leuven Belgium
Tel: +32 16 38 12 11
