Background
Transarterial chemoembolization with drug-eluting microspheres (DEM-TACE) is recommended for patients with BCLC stage B hepatocellular carcinoma (HCC) and stage 0-A unsuitable for curative treatments (i.e. surgery or ablation).
Objective:
This study assessed efficacy and safety, along with hepatobiliary toxicities (HBT) of DEM-TACE using LifePearlTM microspheres, loaded with anthracyclines (doxorubicin or idarubicin)
Methods
Prospective, multi-centre real-life registry.
97 patients diagnosed with unresectable HCC were prospectively enrolled and treated with DEM-TACE using LifePearlTM microspheres loaded with doxorubicin (77%) or idarubicin (23%).
Safety and tolerability were assessed using CTCAE, HBT by CT/MRI scans and tumour response by applying the modified Response Evaluation Criteria in Solid Tumours (mRECIST).
Follow-up was at least 2 years
Results
De Baere et al registry (n=97) demonstrated:
Safety:
Adverse events (AE) were reported in 73.2% of patients, 71% of those being Grade 1–2. Grade ≥ 3 AE, reported in 13.4% of patients, were mainly related to postembolization syndrome.
HBT were observed after 15.5% (29/187) of the DEM-TACE interventions.
The strongest predictor for HBT was previous loco-regional treatment with an odd ratio of 2.65 (95% CI: 1.07–6.61)
Safety was good overall, and HBT rates were even lower than previously reported for microspheres TACE treatments
Efficacy:
Objective response and disease control rates were 81% and 99%, respectively, as the best responses.
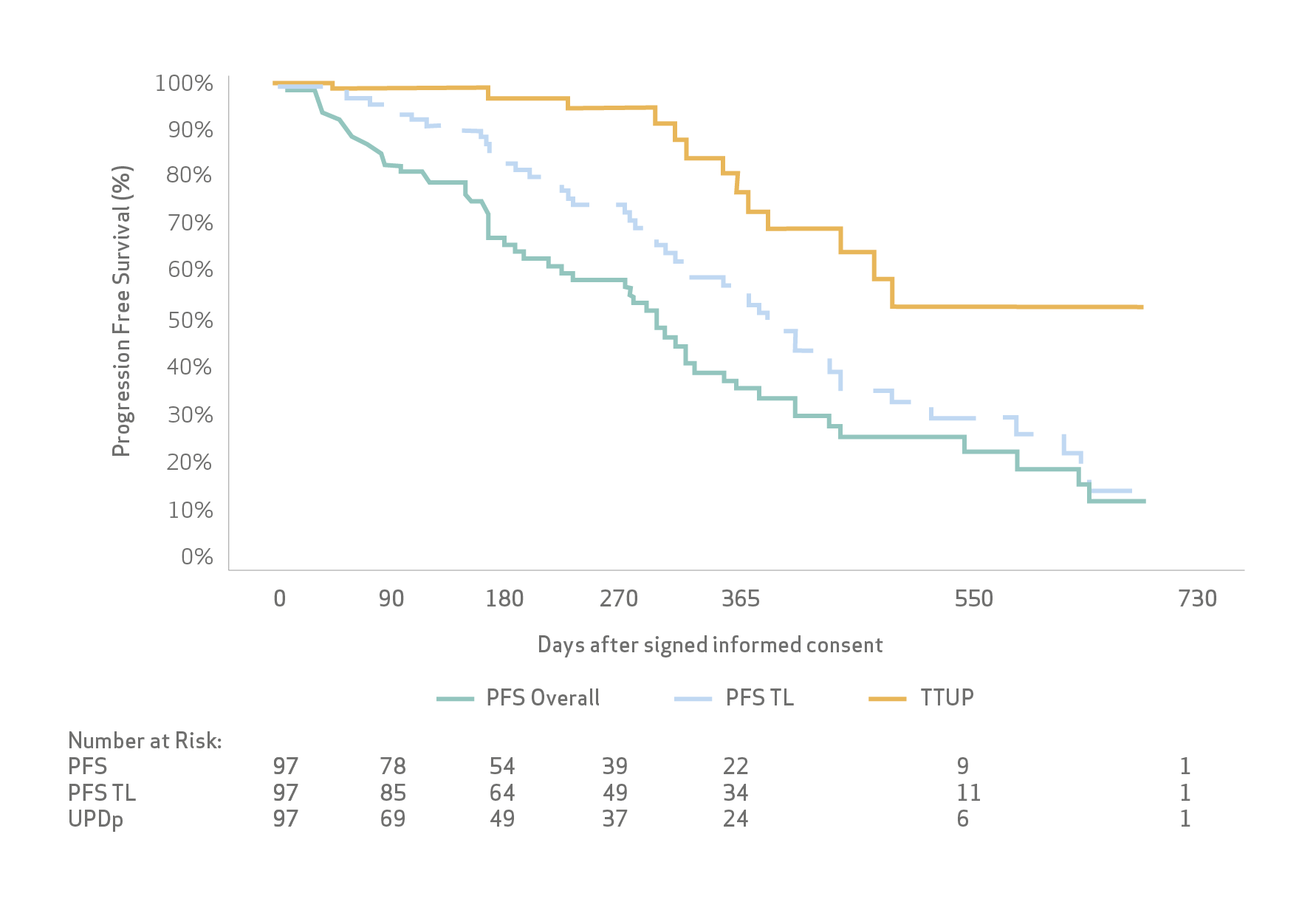
Median progression free survival was 13.7 months (95% CI: 11.3; 15.6)
Median time to TACE-untreatable progression was 16.7 months (95% CI: 12.7; not estimable (n.e.).
Survival rates at one and two years were 81% and 66%, respectively, while the median overall survival (OS) was not reached.
The good safety profile also allowed for successful retreatments à 20% of pts had CR after 3rd or 4th DEM-TACE
Limitations: The main limitations of this study are the absence of a control arm, unselected patient population with multiple comorbidities and treatments following routine clinical practice, resulting in variability of the patient management, timing of imaging follow-up and response evaluation. However, inclusion of all patients eligible for TACE in this study and liberal assessment protocols give a realistic picture of contemporary TACE practice and patient outcomes.
Conclusion
De Baere et al concluded that DEM-TACE using LifePearlTM provides a high tumour response rate in HCC patients. HBT rates, within or below previously reported results for cTACE and DEM-TACE, further confirm a good safety profile for LifePearlTM.
Key Takeaway:
The main findings of De Baere et al are that:
DEM-TACE using anthracycline-loaded LifePearl™ provides a high level of disease control for HCC, within or above the rate previously reported in the literature. This high response rate was obtained irrespective of the type of anthracycline or stage of disease, with acceptable safety.
Overall HBT rate was within or below the previously reported range. Clinical relevance of HBT is still under discussion. A logistic regression analysis did not identify HBT as a predictive factor for OS, and OS, PFS and TTUP did not differentiated between patients with or without HBT in this study.
Another interesting finding of this real-life prospective study is the high proportion of patients in the early stage of disease (BCLC 0 and A) receiving TACE, thus confirming the trend in real-life practice of using DEM-TACE as a bridge to transplant or as downstaging to resection.
The results of this study further confirm the importance of obtaining a high response rate and of aiming for CR, even if several DEM-TACE sessions are necessary. CR also demonstrated to be a key factor in order to obtain longer TTUP and longer OS.
Access to full publication: https://www.mdpi.com/2072-6694/12/11/3405/htm
Use/Indications
European Economic Area Indications for use
LifePearl™ microspheres are indicated for embolization of blood vessels supplying primary hypervascular tumours or metastases in the liver. Note: LifePearl™ microspheres can be loaded with chemotherapeutic drugs. When used for drug loading, drug loading should be done under a physician's direction, choice and responsibility, based on type and dose of drug most beneficial to the patient. LifePearl™ microspheres are compatible with doxorubicin, epirubicin idarubicin and irinotecan. LifePearl™ microspheres can be drug loaded prior to embolization and then, as a secondary action, elute a local, controlled, and sustained dose to the targeted tumour sites after embolization. LifePearl™ microspheres are not available for sale in all countries. This information is provided only in respect to markets where this product is approved or cleared.
This literature summary is not a systemic review. It is only an example of LifePearl microspheres related literatures.
The use of the LifePearl™ devices in combination with drugs is not cleared or approved in the USA by the Food and Drug Administration. LifePearl™ microspheres are not approved in Canada. Please consult the indication of use with the IFU supplemented with the product.
Please contact your Terumo local sales representative for more information. All brand names are trademarks or registered trademarks of TERUMO CORPORATION and their respective owners. Refer to Instructions for Use for Contraindications, Warnings and Precautions.
©2020 MicroVention Europe CE0297
Manufacturer
MicroVention Europe
30 bis, rue du Vieil Abreuvoir
78100 Saint-Germain-en-Laye - France
Tel: +33(0)1 39 21 77 46
Distributor
Terumo Europe N.V.
Interleuvenlaan 40
3001 Leuven Belgium
Tel: +32 16 38 12 11
