Background
Currently, there are several Drug-eluting Microspheres (DEM) available on the market and a new generation of loadable embolics made of polyethylene glycol (PEG) has been developed named LifePearlTM. The clinical data of its use in Hepatocellular carcinoma (HCC) is increasing.
Objective
To evaluate the efficacy and safety profile of transarterial procedures with LifePearl microspheres in the treatment of HCC.
Methods
Single centre retrospective data collection.
Patients with unresectable HCC and liver transplant candidates were treated with LifePearl microspheres loaded with 75 mg of doxorubicin each for a maximum dose of 150 mg per session.
The procedure was performed after super selective catheterization of the tumor-feeding artery, and 1 or 2 vials of LifePearl microspheres,
Tumor response was evaluated at different time points per mRECIST and biochemical safety was collected.
Results
Veloso Gomes et al study (n=302) demonstrated:
A total of 302 patients were treated with LifePearl microspheres. The mean patient age was 66 years ± 10; 142 (47%) had Barcelona Clinic Liver Cancer stage A disease and 134 had (44.4%) stage B disease; 174 (57.6%) had a single HCC tumor, 65 (21.5%) had 2, and 62 (20.9%) had 3 or more.
Median follow-up time was 11.9 months.
Tumor response:
Tumor objective response rate at 1 month was 85.5%.
Progression free survival rate at 12 months was 65.9%
Overall survival rate at 12 months was 93.5%
Patients who received transplants showed a 57.7% rate of complete pathologic response.
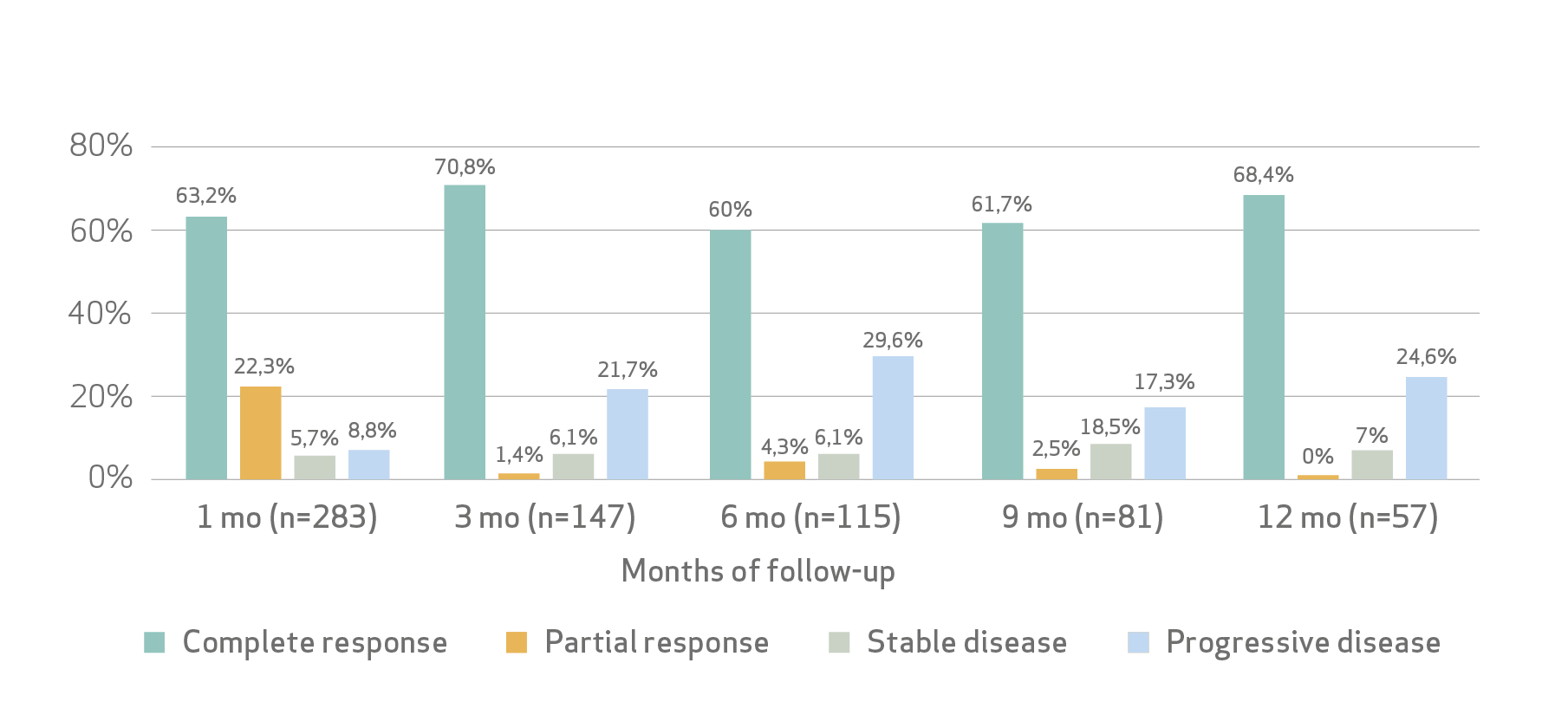
Safety:
The most frequent complications were postembolization syndrome in 18 patients (6%), liver abscess in 5 (1.7%), and puncture site hematoma in 3 (1%).
Biochemical toxicities occurred in 57 patients (11.6%).
Limitations:
Limitations of the present study include the retrospective nature of the study in a single center, the heterogenous nature of the patient sample typical of an HCC cohort, and the lack of evaluation of patient-reported outcomes, which has not been standardized in the authors' practice. Additionally, the study period was too short to allow a robust evaluation of overall survival, particularly because the results in the present cohort were affected by early transplantation complications. Finally, survival analysis should be interpreted with caution, given the absence of matched controls to compare causes of death and comorbidity profiles
Conclusion
Veloso Gomes et al concluded that transarterial treatment procedures with LifePearl Microspheres are effective with a good safety profile for the treatment of unresectable HCC and as a bridge therapy for patients awaiting liver transplantation, achieving a high CR rate at different time points and a low rate of major complications.
Key Takeaway
According to Veloso Gomes et al. transarterial treatment procedures with LifePearl microspheres for the treatment of HCC are effective with a good safety profile, achieving an objective response rate of 85.5%.
LifePearl microspheres can be used as a bridging therapy for transplant candidate patients achieving high tumour response rates.
Access to full publication: https://pubmed.ncbi.nlm.nih.gov/29724521/
European Economic Area Indications for use
LifePearl™ microspheres are indicated for embolization of blood vessels supplying primary hypervascular tumours or metastases in the liver. Note: LifePearl™ microspheres can be loaded with chemotherapeutic drugs. When used for drug loading, drug loading should be done under a physician's direction, choice and responsibility, based on type and dose of drug most beneficial to the patient. LifePearl™ microspheres are compatible with doxorubicin, epirubicin idarubicin and irinotecan. LifePearl™ microspheres can be drug loaded prior to embolization and then, as a secondary action, elute a local, controlled, and sustained dose to the targeted tumour sites after embolization. LifePearl™ microspheres are not available for sale in all countries. This information is provided only in respect to markets where this product is approved or cleared.
This literature summary is not a systemic review. It is only an example of LifePearl microspheres related literatures.
The use of the LifePearl™ devices in combination with drugs is not cleared or approved in the USA by the Food and Drug Administration. LifePearl™ microspheres are not approved in Canada. Please consult the indication of use with the IFU supplemented with the product.
Please contact your Terumo local sales representative for more information. All brand names are trademarks or registered trademarks of TERUMO CORPORATION and their respective owners. Refer to Instructions for Use for Contraindications, Warnings and Precautions.
©2021 MicroVention Europe CE0297
Manufacturer
MicroVention Europe
30 bis, rue du Vieil Abreuvoir
78100 Saint-Germain-en-Laye – France
Tel: +33(0)1 39 21 77 46
Distributor
Terumo Europe N.V.
Interleuvenlaan 40 - 3001 Leuven Belgium
Tel: +32 16 38 12 11
