Background
A considerable proportion of patients at high bleeding risk who undergo drug-eluting stent (DES) implantation are indicated for oral anticoagulation (OAC); however, the optimal duration of antiplatelet therapy after such procedures is yet to be established, especially in combination with OAC.
MASTER DAPT (MAnagement of high bleeding risk patients post bioresorbable polymer coated STEnt implantation with an abbReviated versus prolonged DAPT regimen) was an investigator‑initiated, randomised, open‑label trial conducted in patients at high bleeding risk after the implantation of an Ultimaster™/Ultimaster™ Tansei™ DES. The trial compared abbreviated (1 month) dual antiplatelet therapy (DAPT) with non-abbreviated (3–12 months) DAPT.
The MASTER DAPT trial recruited patients at high bleeding risk regardless of clinical presentation, and is valuable to inform clinical practice.
Objective
In this prespecified subgroup analysis, safety outcomes were assessed in patients who received abbreviated and non-abbreviated DAPT regimens post-implantation of an Ultimaster™ family DES, with or without an indication for concomitant OAC, after 12 months of follow-up.
Methods
A total of 4579 patients at high bleeding risk who had received an Ultimaster™ family DES for acute or chronic coronary syndromes and had no ischaemic events during the first month (30–44 days) after the index procedure were randomised 1:1 to abbreviated or non-abbreviated DAPT. Treatment allocations according to OAC indication are shown in Figure 1.
Figure 1. Treatment allocations according to OAC indication
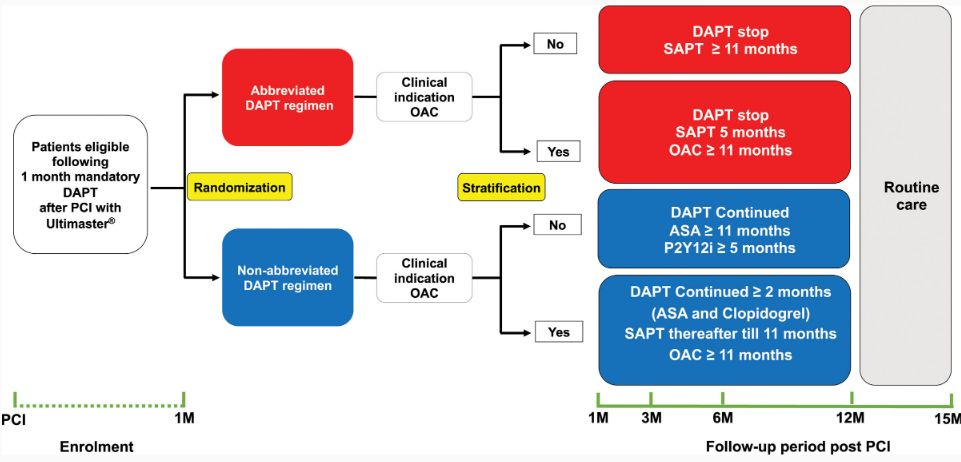 *ASA, acetylsalicylic acid; M, months; P2Y12i, P2Y12 inhibitor; PCI, percutaneous coronary intervention; SAPT, single antiplatelet therapy.
*ASA, acetylsalicylic acid; M, months; P2Y12i, P2Y12 inhibitor; PCI, percutaneous coronary intervention; SAPT, single antiplatelet therapy.
Source: Smits PC et al. Circulation 2021;144:1196–211
Three co-primary outcomes were assessed:
- Net adverse clinical events (NACE) – a composite of death from any cause, myocardial infarction (MI), stroke, and type 3 or 5 bleeding according to the Bleeding Academic Research Consortium (BARC).
- Major adverse cardiac and cerebral events (MACCE) – a composite of death from any cause, MI, and stroke.
- Major or clinically relevant nonmajor bleeding (MCB) – a composite of BARC bleeding type 2, 3, or 5.
Results
Overall, baseline characteristics were similar between groups. Mean age was 76 years, and most (84.2%) of the patients in the OAC group had atrial fibrillation. Of those receiving OAC, 64.9% received a novel oral anticoagulant, and 33.5% a vitamin K antagonist, largely in combination with clopidogrel (98.8%) in the abbreviated DAPT group or acetylsalicylic acid plus clopidogrel (97.4%) in the non-abbreviated DAPT group.
Adherence to the allocated antiplatelet regimen decreased over time and was lower in the abbreviated versus non-abbreviated DAPT arm at 12 months in the OAC group (82.7% vs 95.8%; p<0.001). Clinical outcomes at 12 months are shown in Figure 2:
Figure 2. Kaplan–Meier curves of the three co-primary outcomes at 12 months of follow-up
Net adverse clinical events (NACE)
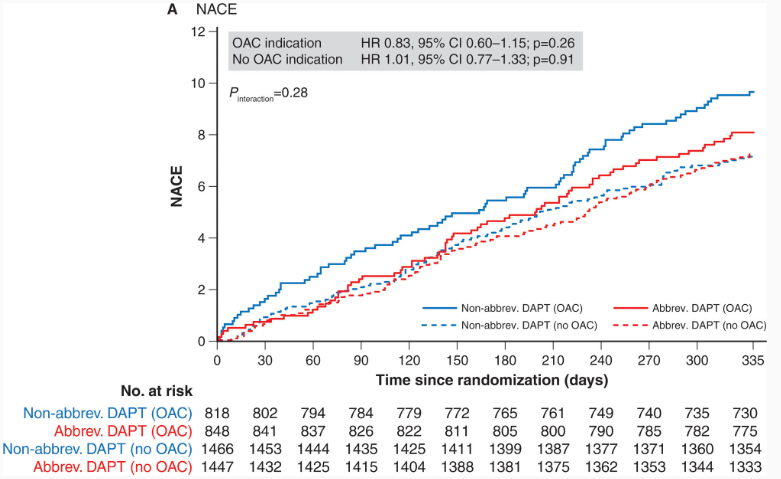
Source: Smits PC et al. Circulation 2021;144:1196–211
– NACE did not differ between patients who received abbreviated and non-abbreviated DAPT
- OAC group: 8.0% versus 9.6%, respectively; hazard ratio (HR): 0.83 (95% confidence interval [CI]: 0.60–1.15); p=0.26
- No OAC group: 7.2% versus 7.1%, respectively; HR: 1.01 (95% CI: 0.77–1.33); p=0.91 (Figure 2A)
Major adverse cardiac and cerebral events (MACCE)
Source: Smits PC et al. Circulation 2021;144:1196–211
– MACCE did not differ between patients who received abbreviated and non-abbreviated DAPT
- OAC group: 5.9% versus 6.7%, respectively; HR: 0.88 (95% CI: 0.60–1.30); p=0.53
- No OAC group: 6.1% versus 5.7%, respectively; HR: 1.06 (95% CI: 0.79–1.44); p=0.67 (Figure 2B)
Major or clinically relevant nonmajor bleeding (MCB)
Source: Smits PC et al. Circulation 2021;144:1196–211
– BARC 2, 3, or 5 bleeding
- OAC group: there were numerical differences between patients who received abbreviated and non-abbreviated DAPT (9.9% vs 11.7%, respectively; HR: 0.83 [95% CI: 0.62–1.12]; p=0.25)
- No OAC group: bleeding events were significantly reduced among patients who received abbreviated DAPT versus nonabbreviated DAPT (4.6% vs 8.1%, respectively; HR: 0.55 [95% CI: 0.41–0.74]; p<0.001) (Figure 2C).
Conclusion
In the largely unselected population of patients at high bleeding risk after coronary stenting with an Ultimaster™ family DES, stopping DAPT 1 month after the procedure was associated with lower bleeding risk, without additional ischaemic risk, both in patients who received OAC therapy and in those who did not. Further research is required to explore the effect of stopping all DAPT after 6 months in patients requiring OAC.
Link to the full publication: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.121.056680
