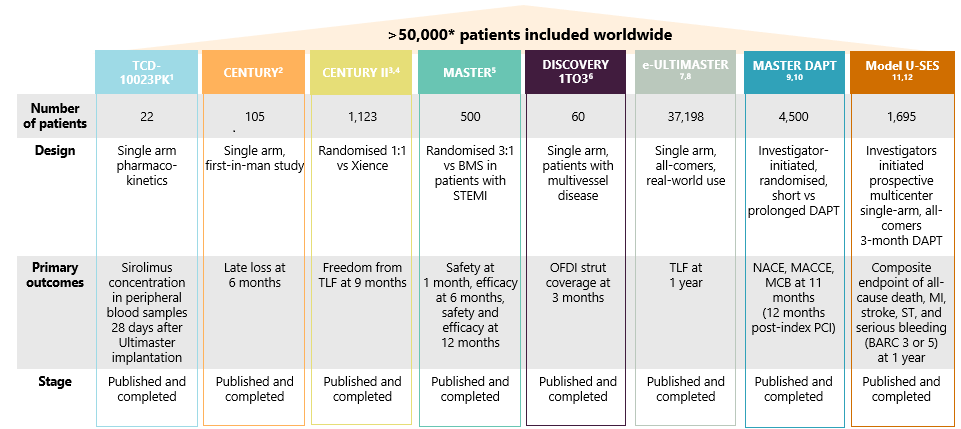
A comprehensive clinical program
Assessment of ‘safety’ refers to
the side-effect profile and clinical events experienced (eg stroke, myocardial infarction,
unplanned revascularisation). BMS, bare-metal stent; DAPT, dual antiplatelet
therapy; MACCE, major adverse cardiac and cerebral events; MACE, major adverse
coronary events; MCB, major or clinically relevant non-major bleeding; NACE,
net adverse clinical endpoints; OFDI, optical frequency domain imaging; PCI,
percutaneous coronary intervention; STEMI, ST-segment elevation myocardial
infarction; TLF, target lesion failure.
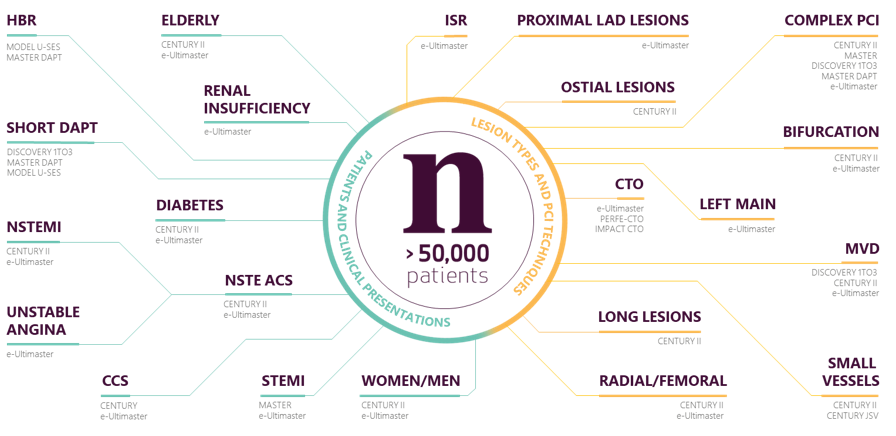
A remarkable 16 CE marked indications
Consistent good safety* data at 1 year
from controlled clinical trial4 and
routine clinical practice8
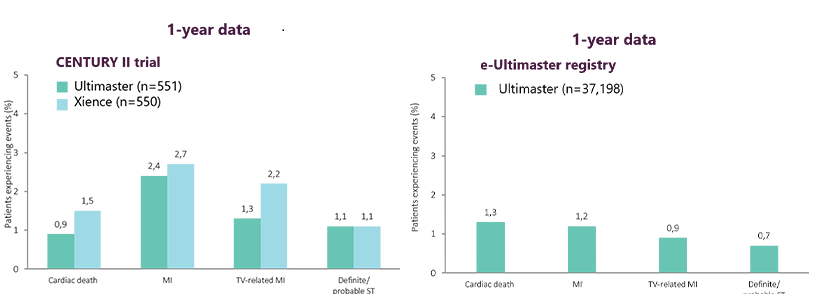
For CENTURY II, patient numbers are from the per-protocol analysis at baseline. p=non-significant, unless otherwise stated.
MI, mycordial infarction; ST, stent thrombosis; TV, target vessel.
*Statements on 'safety are based on cardiac death, MI and ST rates in the referenced study.
Sustained long-term safety* from
controlled clinical trial4
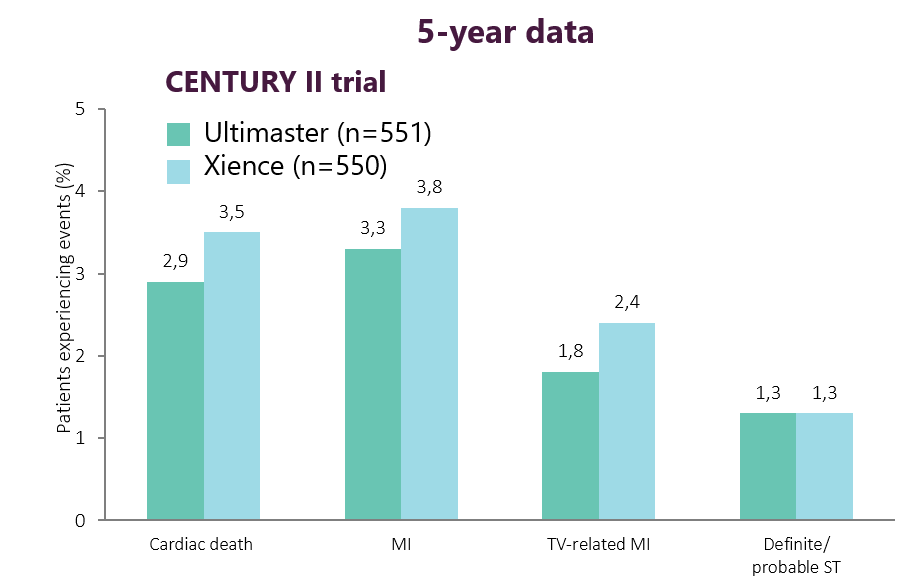
For CENTURY II, patient numbers are from the per-protocol analysis at baseline. p=non-significant, unless otherwise stated.
MI, mycordial infarction; ST, stent thrombosis; TV, target vessel.
*Statements on 'safety are based on cardiac death, MI and ST rates in the referenced study,**data on file.
1-month DAPT for HBR patients
MASTER DAPT is the largest multi-center randomized controlled study to confirm the safety and efficacy of abbreviated DAP in HBR patients following stenting procedures with Ultimaster Family DES. Learn more.

References:
- Stojkovic S et al. Fundam Clin Pharmacol 2014;29:95–105;
- Barbato E et al. EuroIntervention 2015;11:541–8;
- Saito S et al. Eur Heart J 2014;35:2021–31;
- Wijns W et al. EuroIntervention 2018;14:e343–51;
- Valdes-Chavarri M et al. EuroIntervention 2019;14:e1836-42;
- Chevalier B et al. Circ Cardiovasc Interv 2017;10:pii:e004801;
- Mohamed et al. EuroIntervention 2020;16:603-612
- Cimci M et al. Heart 2022;108:1310-1318.
- Frigoli E et al. Am Heart J 2019;209:97-105
- Valgimigli M et al. N Engl J Med 2021;385:1643-1655
- Kozuma K et al. Circ J 2020;85:19-26
- Hioki H et al.J Cardiol 2021;78:107-113
