DES used in Japan in a majority of interventional treatments of myocardial infarction and other coronary events
A stent is a medical implant device used in the treatment of angina pectoris, myocardial infarction, and other coronary events caused by stenosed or blocked blood vessels (coronary arteries) that send nourishment to the heart. In Japan, stents are used in approximately 200,000 cases every year.
After a stent is implanted in a blood vessel, the vessel may become restricted again, causing tissue proliferation or other complications. This is why DES have come into use in a majority of treatments, as the drug can be gradually released to the surrounding tissues, which is expected to reduce such tissue proliferation.
Terumo's original bioresorbable polymer and drug coating technology is expected to help lower the frequency of thrombosis
For Ultimaster, Terumo adopted bioresorbable polymer as the drug coating material and coated only the outer surface that comes into contact with the blood vessel tissues with the polymer and drug. For this reason, Ultimaster is expected to help lower the frequency of stent thrombosis* cases over the long term.
Furthermore, Terumo used cobalt chromium alloy as the material and devised a unique design that makes it easier to navigate the stent through the blood vessels and implant it along the curvature of the vessels. The aim of this design was to reduce stress on the blood vessel walls and improve medical prognosis.

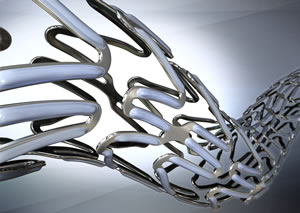
Part of the stent after it has been dilated With Terumo's original drug coating technology,
the drug is released efficiently into the blood vessel
tissues, after the stent is dilated
*Stent thrombosis is a complication that occurs when a blood clot forms inside an implanted stent and constricts or blocks the passageway of the stent.
(Notice) Among the information that Terumo discloses, the forward-looking statements including financial projections are based upon our assumptions using information available to us at the time and are not intended to be guarantees of future events or performance. Accordingly, it should be noted that actual results may differ from those forecasts on projections due to various factors. Factors affecting to actual results include, but are not limited to, changes in economic conditions surrounding Terumo, fluctuations of foreign exchange rates, and state of competition. The market share information in this press release is partly derived from our own independent research.
