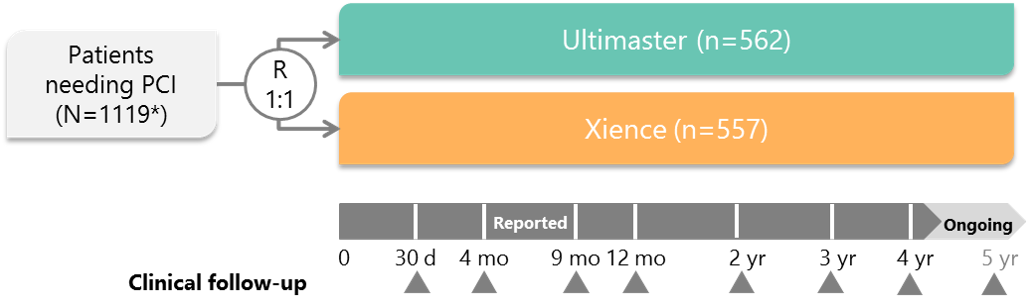
Study design and key features
"Xience" is a product and trademark of the Abbott Group of Companies
* Intention-to-treat population PCI, percutaneous coronary intervention R, randomization
Ultimaster was non-inferior to Xience in terms of the primary endpoint: freedom from TLF at 9 months
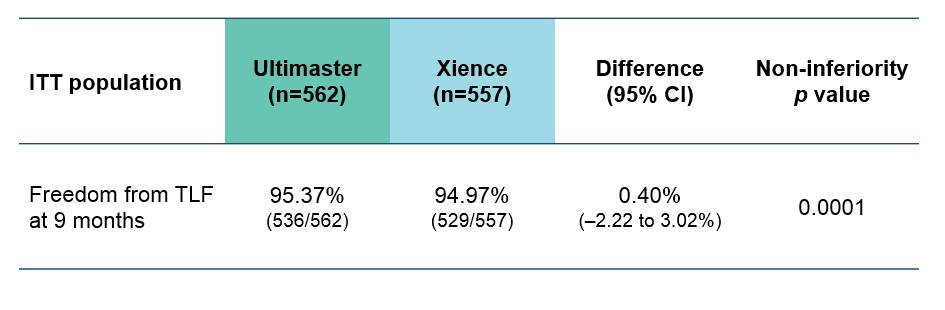

Saito S et al. Presented at EuroPCR 2018, abstract LBT8784.
Data on file at Terumo Europe
TLR, target lesion revascularization
TLF, target lesion failure
CI, confidence interval
MI, myocardial infarction;
NS, not significant;
ST, stent thrombosis;
TV, target vessel.
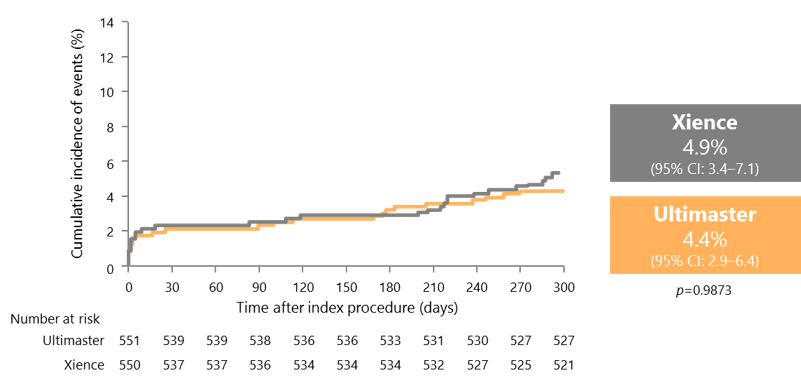
TLF rates similar for Xience and Ultimaster in the first 300 days
Long-term follow-up of CENTURY II: consistent and sustained safety at 1 year and 5 years
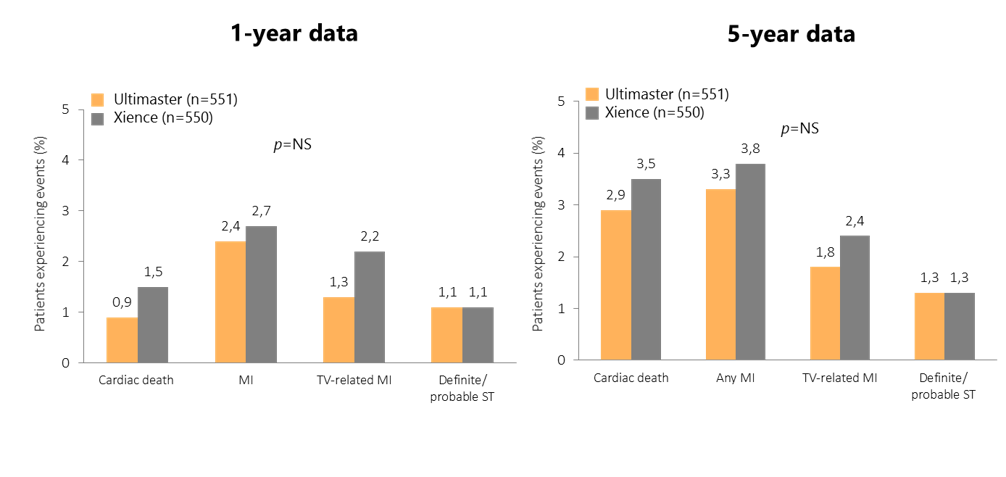
Patient numbers are from the per-protocol analysis at baseline.
CENTURY II included pre-specified assessment of complex subgroups
Bifurcation: clinical evidence from CENTURY II
- There were no significant differences in baseline patient characteristics or bifurcation lesion characteristics between the two groups
- TLF up to 5 years was 7.4% with Ultimaster, compared with 11.1% with Xience (p=0.37)
- Cardiac death at 5-year follow up was significantly lower in the Ultimaster group than in the Xience group (0.0% vs 5.1%; p=0.03)
TLF, target lesion failure.
Orvin K et al. Catheter Cardiovasc Interv 2016;87:1092–100; Chevalier B. Presented at EuroPCR 2018, abstract POS101.
CENTURY II bifurcation subgroup: clinical outcomes up to 5 years
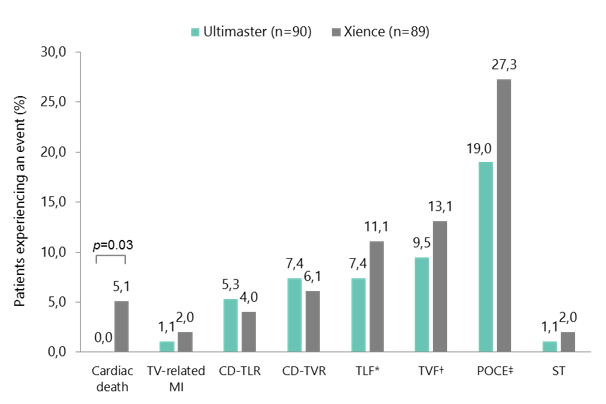
*Composite endpoint of cardiac death, TV-related MI, and CD-TLR. †Composite endpoint of cardiac death, TV-related MI, and CD-TVR. ‡Composite endpoint of all death, any MI, and any coronary revascularisation. CD-TLR, clinically driven target lesion revascularisation; CD-TVR, clinically driven target vessel revascularisation; MI, myocardial infarction; POCE, patient-oriented composite endpoint; ST, stent thrombosis; TLF, target lesion failure; TV, target vessel; TVF, target vessel failure.
Chevalier B et al. Presented at EuroPCR 2018, abstract POS101.
Multivessel disease: clinical evidence from CENTURY II
- There were no significant differences in baseline patient characteristics, lesion characteristics, or procedural characteristics between the two groups
- 69.8% of procedures that used Ultimaster were performed using transradial access, and the overall procedure success rate was 97.8%
- At 5 years, there was a trend towards better clinical outcomes with Ultimaster than with Xience (statistical significance not met)
- Any death: 9.3% vs 10.8% (p=0.60)
- Any MI: 3.1% vs 5.6% (p=0.19)
- Stent thrombosis: 0.9% vs 1.7% (p=0.43)
MI, myocardial infarction.
Iñiguez A et al. Catheter Cardiovasc Interv. 2019. doi: 10.1002/ccd.28224
CENTURY II MVD subgroup: clinical outcomes up to 5 years
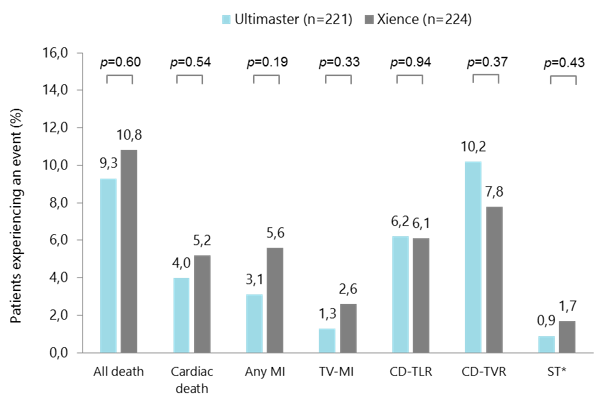
*Definite or probable ST. CD-TLR, clinically driven target lesion revascularisation; CD-TVR, clinically driven target vessel revascularisation; MI, myocardial infarction; MVD, multivessel disease; ST, stent thrombosis; TV, target vessel. Iñiguez A et al. Catheter Cardiovasc Interv. 2019. doi: 10.1002/ccd.28244
CENTURY II MVD subgroup: target lesion failure up to 5 years
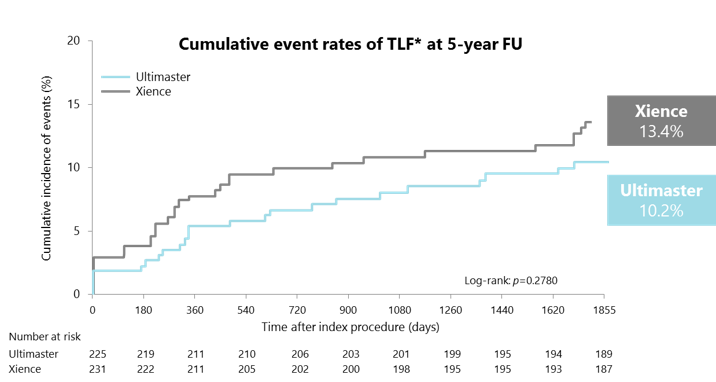
*TLF: composite endpoint of cardiac death, TV-related MI, and clinically driven target lesion revascularisation. FU, follow-up; MI, myocardial infarction; MVD, multivessel disease; TLF, target lesion failure; TV, target vessel. Iñiguez A et al. Catheter Cardiovasc Interv. 2019. doi: 10.1002/ccd.28224.
Acute coronary syndrome: clinical evidence from CENTURY II
- There were no significant differences in baseline patient characteristics, lesion characteristics, or procedural characteristics between the two groups
- In the Ultimaster and Xience groups, 23.0% and 23.1% of patients, respectively, had STEMI, and 76.9% and 76.8%, respectively, had NSTEMI
- Transradial access was used in 73.0% and 71.7% of procedures with Ultimaster and Xience, respectively
- In general, clinical outcomes at 2 years were numerically better with Ultimaster than with Xience
- Any death: 1.5% vs 2.9% (p=0.48)
- Cardiac death: 0.0% vs 2.1% (p=0.10)
- TV-related MI: 0.7% vs 3.6% (p=0.12)
MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; TV, target vessel.
Jimenez VA et al. Cardiovasc Revasc Med 2016;17:355–61; Data on file at Terumo Europe.
CENTURY II ACS subgroup: target lesion failure at 2 years
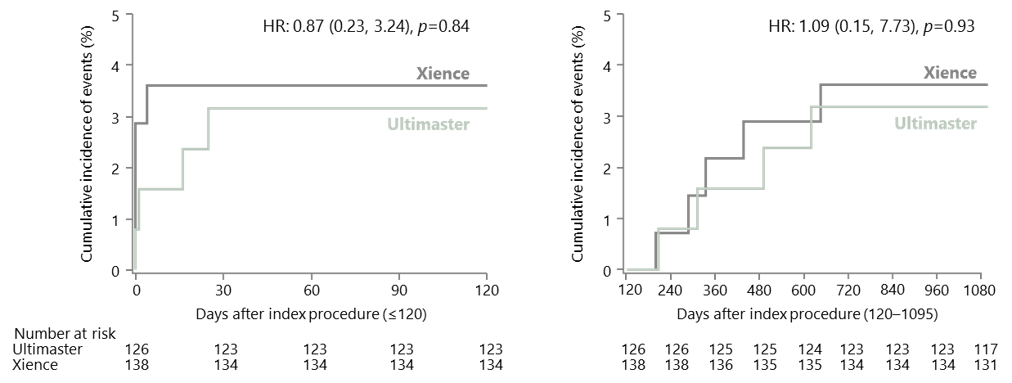
ACS, acute coronary syndrome; HR, hazard ratio.
Jimenez VA et al. Cardiovasc Revasc Med 2016;17:355–61
NSTEMI: clinical evidence from CENTURY II
- In general, there were no significant differences in baseline patient characteristics, lesion characteristics, or procedural characteristics between the two groups
- The number of lesions treated was 1.3 with Ultimaster vs 1.5 with Xience (p<0.02)
- Clinical outcomes with Ultimaster were favourable, with low incidences of cardiac death, TV-related MI, and stent thrombosis
MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; TV, target vessel.
Iñiguez-Romo A. Presented at EuroPCR 2017, abstract OPO704;
CENTURY II NSTEMI subgroup: clinical outcomes at 2 years
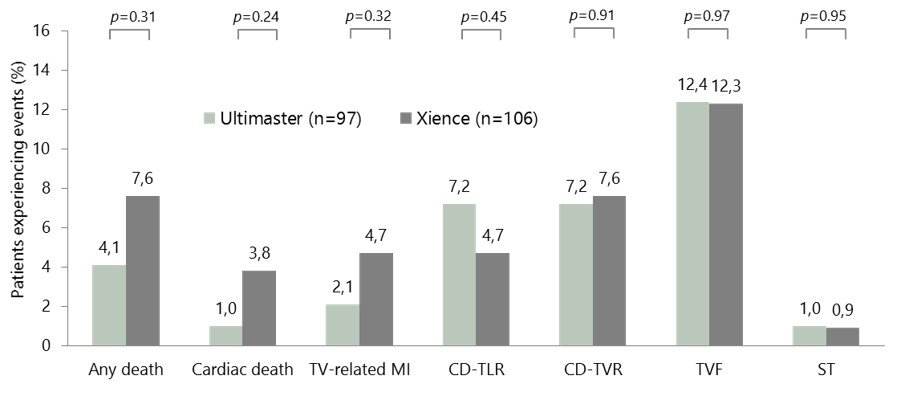
ST=Definite and probable stent thrombosis – 1 subacute ST in each arm.
CD-TLR, clinically driven target lesion revascularisation; CD-TVR, clinically driven target vessel revascularisation; MI, myocardial infarction;
NSTEMI, non-ST-segment elevation myocardial infarction; ST, stent thrombosis; TV, target vessel; TVF, target vessel failure.
Iñiguez-Romo A. Presented at EuroPCR 2017, abstract OPO704.
